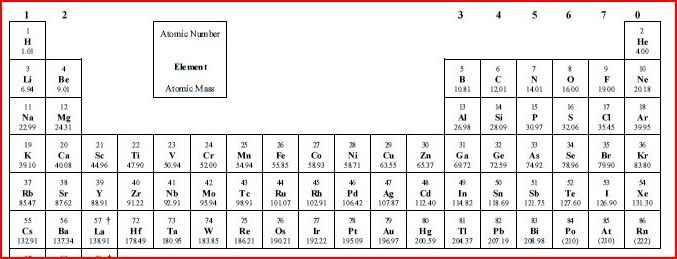
2. Which ions are formed in groups 1, 2 and 3 ?
3. Which elements form ions the easiest ?
4. Which ions are formed in groups 5, 6 and 7 ?
5. Which ions are formed when the transitions metals are ionized ?
7. How can one figure out from the name if a compound is ionic ?
9. What is valence electrons ? How many valence electrons do Cl have (use periodic table) ?
10. How does the strength and length differ for single, double and triple bonds ?
11. How do Lewis diagrams work ?
12. How many covalent bonds do C, N, O, halogens and H form ?
13. Draw the Lewis structure of CH4, H20, NH3, CO2, HCN.
14. Draw the Lewis structure of HF, CF3Cl, C2H6, [NO3]-, SO2, C2H4, [NO]+
16. How can one determine the type of bond from the electronegativity ?
19. What are the rules for polar molecules ?
20. What is intermolecular forces ?
21. what is van der Waals forces ?
22. What is dipole-dipole forces ?
23. What is hydrogen bonding ?
25. Compare boiling point for molecules with van der Waals forces and dipole forces ?
26. Compare boiling point for molecules with hydrogen bonding and dipole forces ?
27. What is metallic bonding ?
28. How does the boiling point depend on the bonding ?
29. How does the volatility depend on the bonding ?
30. How does the conductivity depend on the bonding ?
31. How does the solubility depend on the bonding ?
Download notes on bonding
Go back to the IB chemistry page
Go to the IB physics page
Ionic bond: positive ions (cations) and negative (anions) ions are attracted to each other
and form a continuous ionic lattice.
Group 1 metals form +1 ions, group 2 metals form +2 ions, metals in group 3 form +3 ions.
Examples : Li+, Mg2+, Al3+.
Greater ease of ionisation Li->Cs is due to the increased electron shielding of the nuclear attraction caused by additional inner shells of electrons. The easier atoms are to ionise, the more reactive they will be because less energy is required to ionise them, and so they react faster.
Group 5 will form 3- ions, Group 6 ions will form 2- ions, Group 7 ions will form negative ions.
Examples : O2-, Cl-.
The transitions metals (elements from Ti to Cu, ignoring Sc and Zn) can form multiple ions (i.e. Fe2+, Fe3+) due to proximity of 4s and 3d shells.
The ionic or covalent nature of the bonding in a binary compound is a
result in the difference between their electronegativity. NaCl(s) is ionic, HCl(g)
is (polar) covalent (note: covalent molecules tend to be gases/liquids,
ionic tends to be solid, although network covalent would be solid). In
general, if the difference between electronegativities is greater than
1.7, the bond will be more than 50% ionic.
Check if there is an -ide at the end. Examples: ‘fluoride’,
‘chloride’, ‘bromide’, ‘iodide’ etc , ‘oxide’, ‘sulfide’ etc. or even
nitride or phosphide. These are all ionic compounds.
Covalent bonds are where two atoms each donate 1 electron to form a pair that is shared between the two atoms. Such bonds are generally formed by atoms with little difference in electronegativity, i.e. C, H and O in organic chemistry.
Valence electrons are the electrons in the outer shell. The group number on top
of the periodic table give the number of valence electrons. For Cl it is 7.
For transition elements it is, however, more complicated.

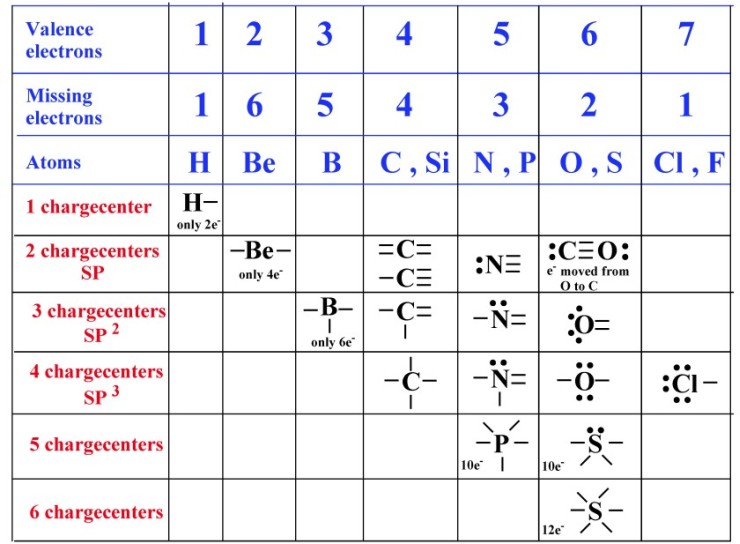
All electrons must be paired in covalent bonding. In Lewis diagram, the
outermost (valence) shell electrons are represented by dots or lines.
The basic idea is that H wants to have one pair of electrons around it and
other atoms wants 4 pairs (8 electrons) around it.
One line = one pair of shared electrons.
Two lines = two pairs of shared electrons.
Three lines = three pairs of shared electrons.
In general, C forms 4 bonds, N forms 3, O forms 2, halogens form 1, H forms 1.
Electronegativity increases across a period, and decreases down each
group. Electronegativity values range from 0.7 to 4, from bottom left to top
right respectively (hydrogen falls B and C with a electronegativity of
2.1 .
When covalent molecules have a difference in electronegativity (between
the two bonding atoms) then the pair will be held closer to the more
electronegative atom. This results in a small negative charge on the more
electronegative atom, and a small positive charge on the other which results
in polar bonds.
The nature of a bond between two atoms can be determined from their electronegativity values
(or position in the periodic table):
Non-polar covalent bond: Atoms have almost the same electronegativity. Examples: H-H , Cl-Cl , C-H
Polar covalent bond: Atoms have slightly different electronegativity (by up to 1.8) . Examples: H-Cl , O-H , C-Cl
Ionic bond: Atoms have very different electronegativity (more than 1.8) . Examples: NaCl
A lone pair is a pair of electrons that do not participate in the covalent bonding.
VSEPR theory: Valence Shell Electron Pair Repulsion. It is a theory which says that the shape of a molecule is determined by the repulsion between the electron pairs in the molecule.
There are polar bonds and there are polar molecules. The polarity of a molecule depends on both
the shape and the polarity of the bonds.
If there are no polar bonds, the molecule is not polar.
If there are polar bonds, but the shape of the molecule is symmetrical, it is not a polar molecule.
If there are polar bonds, and the molecule is not symmetric, then the molecule is polar.
Intermolecular forces are forces between molecules instead of between atoms inside molecules. These forces are weaker than the forces inside molecules.
van der Waal’s forces—Electrons will not be evenly spread around an atom/molecule at any given time, meaning the molecule will have a slight positive charge on one end, and a negative at the other. this temporary state may cause attraction between two molecules, pulling them together (also known as london dispersion forces).
Dipole-dipole forces—Polar molecules, when properly oriented, will attract each other as a result of this. It is stronger than van der Waal’s forces.
Hydrogen bonding—When hydrogen is bonded to nitrogen, oxygen or fluorine, a very strong dipole is formed, making the hydrogen very strongly positive. This hydrogen is then attracted to the lone pairs on other similar molecules (nitrogen, oxygen and fluorine all have lone pairs) forming a hydrogen bond, which is stronger than van der Waal’s or dipole-dipole (but weaker than covalent bonding inside molecules).
Higher Mr gives a higher boiling point in molecules with van der Waals forces.
Dipole forces are stronger than van der Waals forces and therefore give higher boiling point.
Hydrogen bonding is stronger than dipole forces and therefore give higher boiling point.
Metallic bonding: The metal atoms lose their outer electrons which
then become delocalized, and free to move throughout the entire metal.
These negative delocalized electrons hold the metal cations together
strongly. Since these electrons can flow, atoms with metallic bonding
exhibit high electrical conductivity. Unlike ionic bonding, distorting
the atoms does not cause repulsion so metallic substances are ductile
(can be stretched into wires) and malleable (can be made into flat
sheets). The free moving electrons also allow for high thermal
conductivity, and the electrons can carry the heat energy rather than it
being transferred slowly through atoms vibrating.
Melting and boiling points:
Ionic bonding: High
Metallic bonding: High
Covalent molecular bonding: Low
Volatility:
Ionic bonding: Not volatile
Metallic bonding: Not volatile
Covalent molecular bonding: Volatile
Conductivity:
Ionic bonding: Do not conduct when solid, do conduct when molten or dissolved in water.
Metallic bonding: Conduct
Covalent molecular bonding: Polar molecular substances conduct, non-polar ones do not
Solubility:
Ionic bonding: Ionic substances generally dissolve in polar solvents (like water).
Metallic bonding: Metallic substances are generally not soluble.
Covalent molecular bonding: Non-polar molecules are generally soluble in non-polar solvents, and
polar in polar.