
1 - What is a negative charge center ?
2 - How does the shape of the atom depend on the charge centers ?
3 - There are two types of covalent bonds. What is the difference ?
4 - What types of bonds are there in single, double and triple bonds ?
5 - How does the strength of different bonds vary ?
6 - Covalent bonds sometimes involve excitation of an atom. What does that mean?
8 - How does the shape of a molecule depend on the hybridization ?
10 - What is delocalisation of electrons ?
11 - Give examples of molecules with delocalisation of electrons ?
12 - What properties do delocalized electrons give ?
13 -
What is a resonance hybrid ?
Download notes on bonding
Go back to the IB chemistry page
Go to the IB physics page
A negative charge center is lone pair or a bonding pair. A double or triple bonding counts as one negative charge center. The shape of a molecule depends on the charge centers around the central atom and not the charge centers around the other atoms.
The charge centers around the central atom will repulse each other:
lone pair - lone pair have a strong repulsion
lone pair - bonding pair have a medium repulsion
bonding pair - bonding pair have a weak repulsion
Two charge centers: linear shape - Look here !
Three charge centers:if all are bonding pairs then one gets a triangular shape in one plane - Look here !
Three charge centers:if two are bonding pairs and one lone pair then one gets a bent shape in one plane - Look here !
Four charge centers:if all are bonding pairs then one gets a tetrahedral shape. - Look here !
Four charge centers:if there is one lone pair then one gets a pyramidal shape. - Look here !
Four charge centers:if there is two lone pairs then one gets a bent V-shape. - Look here !
Five charge centers:Look here !
Six charge centers:Look here !
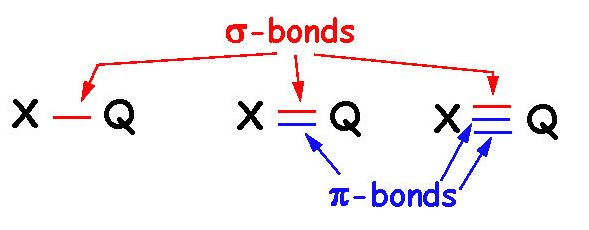
Sigma bonds are bonds between two atoms where the bond is symmetric around the line between the two nuclei of the atoms.
The sigma bond can be between two s-orbitals, one s and one p, or two p-orbitals.
Pi bonds are those which are not symmetric around the line between the two nuclei of the atoms. This happens when
two p-orbitals overlap sideways.
Single bonds: Always sigma bonds.
Double bonds: One sigma bond + one pi bond.
Triple bonds: One sigma bond + two pi bonds.
Triple bonds are stronger than double bonds that are stronger than single bonds.
Sigma bonds are stronger than pi bonds.
Carbon has the in the outer shell 2 electrons in one s-orbital and 2 electrons in two p-orbitals. When it bonds to other atoms it likes to move on electron from s to p so that it has 4 electrons in 4 different orbitals. This is called excitation.
A single atom has certain orbitals. A single Carbon atom has for example 1s2 2s2 2p2.
However, when a Carbon atoms bonds to other atoms, for example 4 hydrogen atoms to form CH4, then new orbitals
are created which are all the same. The bonds are stronger with these hybrid orbitals.
sp3 hybridization: If an atom is bonding by using electrons from one s orbital and three p orbitals.
sp2 hybridization: If an atom is bonding by using electrons from one s orbital and two p orbitals.
sp hybridization: If an atom is bonding by using electrons from one s orbital and one p orbitals.
sp hybridization = 2 negative charge centers: Linear shape
sp2 hybridization = 3 negative charge centers: planar triangular (0 lone pairs) or V-shaped (1 lone pair).
sp3 hybridization = 4 negative charge centers: tetrahedral (0 lone pairs), pyramidal (1 lone pair) or V-shaped (2 lone pair).
Delocalized electrons are shared between more than one bonding position. They spread out over a larger area in a molecule than the typical bonding pairs of electrons. The delocalisation of these pi electrons (which is effectively what happens) makes the molecule more stable (as evidenced by lower energy) and gives the bonds a shorter length than would be expected. The classic example is benzene or O3.
Delocalisation creates bonds that are equal and with a length and strength in between that of a single and double bond.
Delocalisation creates molecules that are more stable since the electrons are spread out which makes the repulsion between them smaller.
Delocalisation increases electrical conductivity so that molecules with delocalized electrons can conduct electricity also in the solid state.
When a particular molecule can be represented as several different
ways (different Lewis structures) the actual shape is generally not
actually any of these, but a hybrid of all of them. This is called
a resonance hybrid.