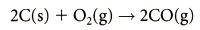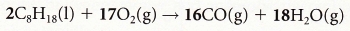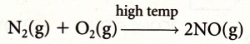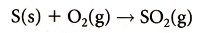Environmental Chemistry - SL & HL E.1 - Air Pollution - questions
E.1.1 -
What does it mean that a source of pollution is anthropogenic ?
E.1.2 -
What are particulates ?
E.1.3 -
What are VOCs ?
E.1.4 -
What are aerosols ?
E.1.5 -
What is anaerobic decomposition ?
E.1.6 -
Describe the main sources of carbon monoxide,
oxides of nitrogen, oxides of sulfur,
particulates and volatile organic compounds in the atmosphere.
E.1.7 -
List all the current methods for the reduction of air pollution !
E.1.8 -
Explain the methods for the reduction of Carbon Monoxide !
E.1.9 -
Explain the methods for the reduction of Nitrogen Oxides !
E.1.10 -
Explain the methods for the reduction of Sulfur Oxides !
E.1.11 -
Explain the methods for the reduction of Particulates !
E.1.12 -
Explain the methods for the reduction of Volatile Organic Compounds !
E.1 - Air Pollution - Exam questions
E.10 - Smog - questions
E.10.1 -
What is a free radical ?
E.10.2 -
What are the two main types of smog ?
E.10.3 -
What are the primary pollutants in the two main types of smog ?
E.10.4 -
State the source of these primary pollutants !
E.10.5 -
What are the conditions necessary for the formation of photochemical smog ?
E.10.6 -
How are the secondary pollutants in photochemical smog formed.
E.10.7 - Explain
E.10.8 -
How can a thermal inversion be created ?
E.10.9 -
What are the bad effects of photochemical smog ?
E.10 - Smog - Exam questions
E.2 & E.11 - Acid Deposition - questions
E.2.1 -
All rain is naturally acidic. Why ?
E.2.2 -
What is the definition of acid rain ?
E.2.3 -
State what is meant by the term acid deposition.
E.2.4 -
What causes acid deposition ?
E.2.5 -
Discuss the effects of acid deposition as well as possible methods to counteract them.
E.11.1 -
Describe the mechanism of acid deposition caused by the oxides of nitrogen and oxides of sulfur.
E.11.2 -
Explain the role of ammonia in acid deposition.
E.2 & E.11 - Acid Deposition - Exam questions
E.3 - Greenhouse Effect - questions
E.3.1 -
Describe the greenhouse effect.
E.3.2 -
Why are some gases greenhouse gases and some are not ?
E.3.3 -
List the main greenhouse gases and their sources and discuss their relative effects.
E.3.4 -
Discuss the influence and effects of increasing amounts of greenhouse gases in the atmosphere.
E.3 - Greenhouse Effect - Exam questions
E.4 & E.9 - Ozone Depletion - questions
E.4.0 -
Describe the regions of the atmosphere and where the ozone layer is.
E.9.1 -
Explain the dependence of O2 and O3 dissociation on the wavelength of light.
E.4.1 -
Describe the formation and depletion of ozone in the stratosphere by natural process.
E.4.2 -
List the ozone-depleting pollutants and their sources.
E.9.2 -
Describe the mechanism in the catalysis of O3 depletion by CFCs and NOx .
E.4.3 -
Discuss the alternatives to CFCs in terms of their properties.
E.9.3 -
Outline the reasons for greater ozone depletion in polar regions.
E.4 & E.9 - Ozone Depletion - Exam questions
E.5 - Dissolved Oxygen In Water - questions
E.5.1 -
What is ppm ?
E.5.2 -
Discuss dissolved oxygen levels in water !
E.5.3 -
Explain what BOD is !
E.5.4 -
How can one measure BOD ?
E.5.5 -
What is the difference between Aerobic and Anaerobic decomposition ?
E.5.6 -
Distinguish between aerobic and anaerobic decomposition of organic material in water.
E.5.7 -
What does eutrophication mean ?
E.5.8 -
Describe the process of eutrophication and its effects.
E.5.9 -
Describe the source and effects of thermal pollution in water.
E.5 - Dissolved Oxygen In Water - Exam questions
E.6 - Water Treatment - questions
E.6.1 -
List the primary pollutants found in waste water and identify their sources.
E.6.2 -
Outline primary, secondary and tertiary stages of waste water treatment, and state the substance that is removed during each stage.
E.6.3 -
Discuss the methods for tertiary sewage treatment !
E.6.4 -
Evaluate the process to obtain fresh water from sea water using multi-stage distillation and reverse osmosis.
E.6.5 -
Discuss advantages and disadvantages of different anti-bacterial treatments.
E.6 - Water Treatment - Exam questions
E.7 - Soil - questions
E.7.1 -
What does soil consist of ?
E.7.2 -
What is SOM ?
E.7.3 -
What is humus ?
E.7.4 -
Discuss different types of soil degradation !
E.7.5 -
How does SOMs prevent soil degradation and discuss the functions of SOMs !
E.7.6 -
What are advantages and disadvantages of tillage ?
E.7.7 -
List common organic soil pollutants and their sources.
E.12 - Water and Soil - questions
E.12.1 -
What is the solubility product constant ?
E.12.2 -
What does the value of the Ksp indicate ?
E.12.3 -
What factors does Ksp depend on ?
E.12.4 -
How can one remove heavy metals in water ?
E.12.5 -
How does the addition of more hydrogen sulfide change the concentration
of heavy metal ions in a saturated solution ?
E.12.6 -
How do you solve problems relating to the removal of heavy-metal ions by chemical precipitation ?
E.12.7 -
What is Silica ?
E.12.8 -
Why does clay attract positive ions to its surface ?
E.12.9 -
Describe cation-exchange in the soil !
E.12.10 -
State what is meant by the term cation-exchange capacity (CEC) and outline its importance.
E.12.11 -
Discuss the effects of soil pH on cation-exchange capacity and availability of nutrients. What pH does different cations need ?
E.8 - Waste - questions
E.8.1 -
Outline and compare various methods for waste disposal.
E.8.2 -
Describe the recycling of metal, glass, plastic and paper products, and outline its benefits.
E.8.3 -
Describe the characteristics and sources of different types of radioactive waste.
E.8.4 -
Compare the storage and disposal methods for different types of radioactive waste.
Go back to the IB chemistry page
Go to the IB physics page
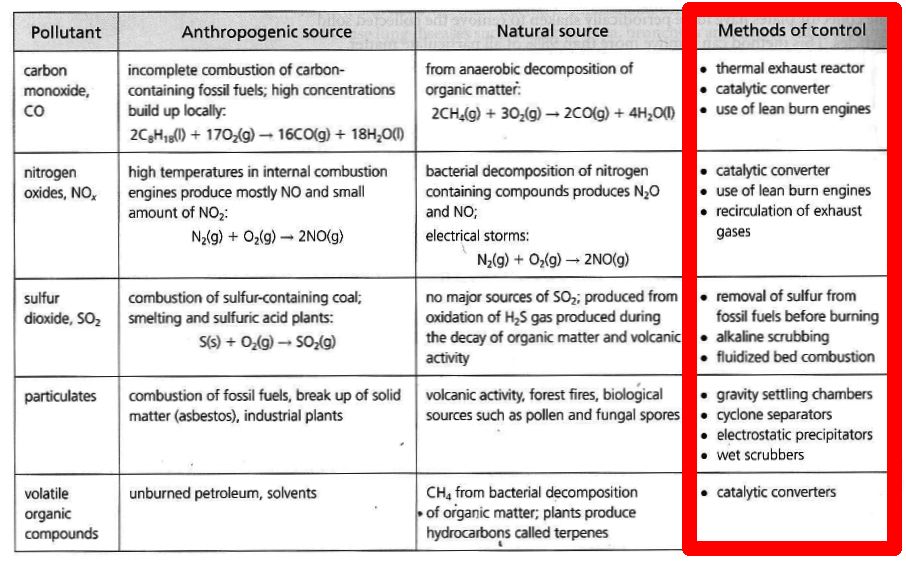
E.1 - Air pollution
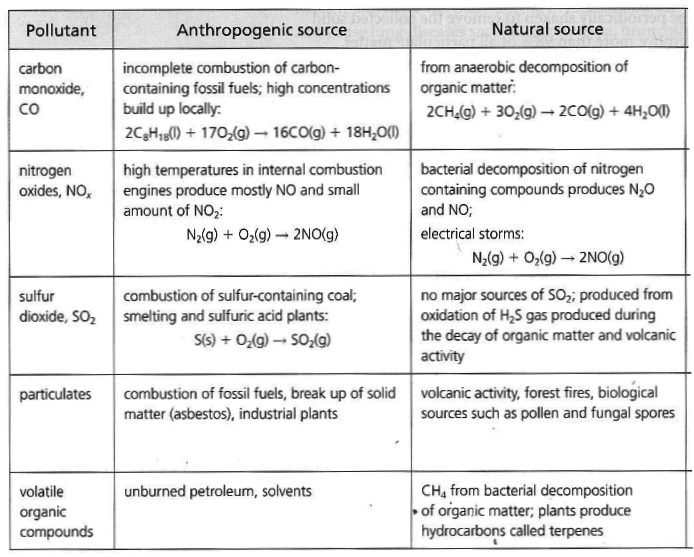
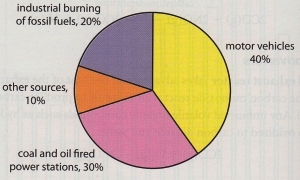
SOURCES
Carbon Monoxide: Nitrogen Oxides: 2 , N2 O).
Anthropogenic sources of nitrogen oxides in the atmosphere are
motor vehicles, industrial burning of fossil fuels, coal and oil fired power stations:Sulfur Oxides: 2 ) and Sulfur Trioxide (SO3 ). Particulates: Volatile Organic Compounds:
METHODS FOR REDUCTION OF AIR POLLUTION
Carbon Monoxide: 1. Thermal Exhaust reactor: The heat of the exhaust gases is used to make carbon monoxide react with more air so that
it is converted to carbon dioxide:
2CO + O2 → 2CO2 2. Catalytic converters: Hot exhaust gases are passed over a platinum-based catalyst which converts carbon monoxide to carbon dioxide:
2CO + O2 → 2CO2 3. Lean burn engines: If more air is added to the combustion process in an engine then carbon dioxide is produced instead of carbon monoxide. However,
this decreases the power of the engine. Compare incomplete combustion of octane when CO is produced with complete combustion of octane when CO2
is produced:
Nitrogen Oxides: 1. Recirculation of exhaust gases: By sending the exhaust gases back into the engine one can LOWER its operating temperature and this reduces
the amount of nitrogen oxide that is produced.2. Catalytic converters: Hot exhaust gases are passed over a platinum-based catalyst which not only converts carbon monoxide to carbon dioxide
but which also converts nitrogen monoxide into nitrogen gas:
2CO + 2NO → 2CO2 + N2 3. Lean burn engines: If more air is added to the combustion process in an engine then there is less CO and nitrogen oxides being produced.
Sulfur Oxides: 1. Removal of sulfur from fossil fuels: The sulphur in coal can be removed by crushing it and washing with water. The heavy metal sulfides
then sinks to the bottom.2. Alkaline scrubbing: Exhaust gases containing SO2 are mixed with an alkaline such as calcium oxide (CaO) and solid CaSO3 and CaSO4 waste is produced.
3. Fluidized bed combustion: Here the coal (instead of the exhaust gases) is mixed with powdered calcium oxide (CaO) before burning it and SO2 is removed
while solid CaSO3 and CaSO4 waste is produced.
Particulates: 1. Gravity settling chambers: Dirty air is sent to a chamber where particulates fall to the bottom thanks to gravity.
2. Cyclone separators: The exhaust gases are spun rapidly so that the particulates are thrown outwards were they are collected.3. Electrostatic precipitators: The dirty air is passed through a high voltage electric field that makes the particulates negatively charged.
The air is then sent pass positively charged plates where the particulates gets stuck.4. Wet scrubbers: Wet scrubbers remove dust particles by capturing them in water droplets. The water droplets with the particulates are then removed.
Volatile Organic Compounds: 1. Catalytic converters: Hot exhaust gases are passed over a platinum-based catalyst which oxidizes the hydrocarbons to
carbon dioxide and water.
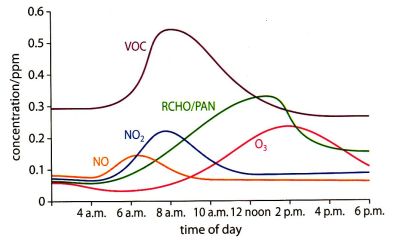
E.10 Smog
Photochemical smog and "pea soup smog".
PRIMARY POLLUTANTS: Photochemical smog: Nitrogen monoxide (NO) that is created when nitrogen and oxygen are combined at high temperature
(N2 + O2 → 2NO) and VOCs from unburnt petroleum such as RH and RCH3 ."Pea soup" smog: Sulfur dioxide (SO2 ) and carbon particulates (C).
Photochemical smog: Traffic exhaust fumes"Pea soup" smog: Burning of coal to heat houses (does not happen much anymore).
CONDITIONS REQUIRED: 1. Thermal inversion: Normally air temperature goes down with increasing altitude and since warm air filled with pollution has a lower density it
goes up and disappears in the atmosphere. In some cases a thermal inversion can occur over a city. Warm air is then trapped in a layer and acts
as a lid that traps the pollutants.
2. Dry sunshine: Sunlight is needed for nitrogen dioxide to participate in photochemical reactions that produces free radicals .
SECONDARY POLLUTANTS: 3 2 → 2NO2 2 --(UV light)--> NO + O-radical 2 + O-radical → O3
A thermal inversion occurs naturally in the stratosphere. Ozone absorbs high energy UV radiation which leads
to an increase in the kinetic energy of the gas particles and thus the temperature of the gas molecules.
Effects of smog:
E.2, E.11 - Acid Deposition
All rain is acidic because carbon dioxide in air reacts with water so that carbonic acid is produced:2 + H2 O → H2 CO3
Acid rain has a pH < 5.6 .
Acid deposition refers to the process by which acidic particles, gases and precipitation leave the atmosphere.
It is a more general term than acid rain and extends to pollution in the absence of water. It is divide up into:Wet deposition: acid rain, snow and fog.Dry deposition: acid gases and particles.
Acid deposition is caused by sulfur dioxide from power plants and nitrogen oxide from cars.SULFUR DIOXIDE NITROGEN DIOXIDE
Acid deposition has many effects on materials, plant life, water and human health.+ and K+ ions
from the soil and releases the dangerous Al3+ ions
from rocks into the soil. Without these essential nutrients, plants starve to death. 3+ ions "trapped" in the rocks are dissolve under acidic conditions. eutrophication .
Control Strategies
Acid deposition can be controlled by reducing the level of emissions of nitrogen and sulfur oxides.
Other possible solutions are to switch to alternative energy sources, such as
wind, solar or tidal energy, or to reduce the demand for fossil fuels by using more public transport
or more efficient energy transfer systems.
NITRIC ACID IS FORMED FROM NITROGEN DIOXIDE
This is what happens in an engine:
N2 + O2 → 2NO*2 → 2NO2 2 → NO* + O*2 O + O* → 2OH*2 + O* → O3 3 + H2 O → 2OH* + O2 2 2 + OH* → HNO3 SULFURIC ACID IS FORMED FROM SULFUR DIOXIDE
HO* + SO2 → HOSO2 *2 * + O2 → HO2 + SO3 3 + H2 O → H2 SO4
Ammonia is present in the atmosphere from both natural and synthetic sources. It is produced naturally by animal livestock and by
the action of certain bacteria and also from artificial fertilizers. The weak base ammonia reacts
with the strong acids present in acid to produce ammonium sulfate and ammonium nitrate.
E.3 - Greenhouse Effect
Greenhouse gases allow the passage of incoming solar short-wave radiation but absorb the longer-wavelength radiation from the earth.
Water - H2 O
1. Its main source is evaporation of oceans and lakes.the major greenhouse gas. Carbon Dioxide - CO2
1. Its main sources are burning of fossil fuels and biomass.the major contribution to global
warming. Methane - CH4
1. Its main sources are anaerobic decay of organic matter and farming.Chlorofluorocarbons - CFCs
1. Its main source is as a refrigerant.Ozone - O3
1. Its main source is as a secondary pollutant in photochemical smog.Dinitrogen Oxide - NO2
1. Its main sources are fertilizers.
Increasing greenhouse gases could increase the earth’s natural greenhouse effect and lead to global warming.
In the last 50 years the amount of CO2 has increased and the average temperature has increased.
If this continuous it could have the following effects:
E.4, E.9 - Ozone Depletion
The energy of a light photon is given by E = hf = hc/λ so a small wavelength (λ) means high energy.2 has a bond order of 2 (a double bond), therefore it is more difficult to break. This means that it will require more energy to do so,
so a shorter wavelength (<242 nm).2 + UV (<242 nm) → 2O*3 , due to its resonant structure, has a bond order of 1.5, meaning it is less difficult to break than the double bond in O2 .
This means that it will require less energy to do so, meaning a longer wavelength (<330 nm).3 + UV (<330 nm) → O2 + O*
The ozone layer occurs in the stratosphere between 12km and 50 km from the surface of the Earth.
Stratospheric ozone is in dynamic equilibrium with oxygen and is continually being formed and decomposed.
When this happens dangerous ultraviolet light is being absorbed and the stratosphere is warmed up. Both
are essential for life on earth.2 + UV → 2O*2 + O* → O3 3 + UV → O2 + O*3 + O* → 2O2 The Chapman cycle:
CFCs - Chlorofluorocarbons: These were previously used as in aerosols, refrigerants, foaming agents, plastics and cleaning solvents.
Unfortunately, these molecules can destroy the ozone layer by producing free Cl-radicals that acts as catalysts
in a process where ozone is turned into oxygen gas. Because they are catalysts the Cl-radicals themselves are
not destroyed in the process and one Cl* can destroy many O3 .NOx - Nitrogen oxides: These are produced by cars and jet aircrafts.
NOx* radicals can also react catalytically with O3 .
FREON - CCl2 F2
Formation of Cl*:
CCl2 F2 + UV → CClF2 * + Cl*
Destruction of ozone:
Cl* + O3 → ClO* + O2
Regeneration of Cl*:
ClO* + O* → Cl* + O2
NITROGEN MONOXIDE - NO
Formation of NO* (the photochemical reaction):
NO2 + UV → NO* + O*
Destruction of ozone:
NO* + O3 → O2 + NO2 *
Regeneration of NO*:
NO2 * + O* → NO* + O2
0. Chloro Fluoro Carbons - CFCs: Non-flammable. Non-toxic. Used in aerosols and as refrigerants in refrigerators and air
conditioners. The Cl-atom destroys the ozone layer.1. Hydro Carbons: Flammable. Toxic. Used in refrigerators.
The Cl-atom has been replaced by a H-atom and the hydrocarbons do not destroy the ozone layer.2. Fluoro Carbons: Non-flammable. Non-toxic. The strong C-F bond makes them stable to ultraviolet light.
Do not destroy the ozone layer.3. Hydro Fluoro Carbons - HFCs: Non-flammable. Low toxicity. No Cl-atoms. Do not destroy the ozone layer.4. Hydro Chloro Fluoro Carbons - HCFCs: Non-flammable. Moderate toxicity. They contain Cl-atoms but most molecules
are destroyed in the lower atmosphere before reaching the ozone layer.
Destroy the ozone-layer 30 times less than CFCs.
A hole in the ozone layer is found above Antarctica. Depletion is seasonal with the largest holes occurring during the early spring (October/November).
This decrease is due to chemicals produced by man.2 which forms a “chlorine reservoir.”2 → Cl* + Cl*
E.5 - Dissolved Oxygen In Water
ppm stands for Parts Per Million. In case of pollutants in water this means the number of grams of pollutants in 1000 kg of water
or in one m3 of water.
The level of dissolved oxygen in water is one of the most important indicators of water quality. The maximum amount of oxygen one can have
in water is 9 ppm at 20 o C. Fish needs 3 ppm to survive.
BOD = Biological Oxygen Demand. It is a measure of the dissolved oxygen (in parts per million) required to decompose all organic waste
and ammonia in water biologically over a 5 day period at 20C. The wastes demand oxygen to be decomposed. It
measures the level of organic pollution in water. Fish cannot survive when the BOD is greater than the oxygen content.
One measure the dissolved oxygen in a water sample (I) and let it stay in a dark place for 5 days and then measure the dissolved oxygen level again (F).
The BOD is then calculated as:
Aerobic decomposition means decomposition with oxygen and Anaerobic decomposition means decomposition without oxygen.
Aerobic Decomposition: If there’s sufficient oxygen present in the water, organic matter is broken down by microbes aerobically.
The organic compounds are oxidized.2 4 3 - 3 4 2- 2 S
Nitrates from fertilizers and phosphates from detergents can accumulate in lakes and streams.
These nutrients can increase the growth of plants and algae. This impacts the BOD because
if plant growth increases too fast and the dissolved oxygen is not sufficient to decompose all organic material
and waste by aerobic decomposition, anaerobic decomposition will occur. More species will
die as a result of the anaerobic decay. The lake will become stagnant and devoid of life.
If water is heated by for example electric power stations, the solubility of oxygen in the water decreases so the amount of dissolved oxygen goes down..
At the same time, fish are cold-blooded, so as the temperature of the water increases, their metabolism increases and they need more oxygen.
So fish and other organisms may die if water is heated.
E.6 - Water Treatment
Dioxins: Pesticides: Polychlorobiphenyls (PCBs): Nitrates: 3 - Heavy metals:
Primary Treatment: the removal of large solids
1. Bar screens: these remove large objects and debris from the surface of the water and remove floating solids.Secondary Treatment: the removal of organic materials using microbes
3. Some of the sludge is mixed with the waste water in a process called the activated sludge process. The idea is that the
bacteria in the sludge will oxidize and break down most of the organic matter.Tertiary Treatment: removal of the remaining organic materials & metal ions, nitrates and phosphate ions.
6. There are different tertiary treatment methods: precipitation, ion exchange, biological methods, activated carbon, distillation, osmosis.
Anti-bacterial Treatment: removal of bacteria.
7. The most common method to kill bacteria in the water is with chlorine but ozone can also be used.
Tertiary Treatment
PRECIPITATION 2+ + H2 S → CdS + 2H+ ACTIVATED CARBON ION EXCHANGE BIOLOGICAL METHODS 3 - (aq) → N2 (g) + 3O2 (g)
In distillation, sea water is pumped into a reservoir, at which point it is heated.
It is then passed into an evacuated chamber where it boils. The salt is left as a salty brine, which is then pumped out
while the steam goes to a condenser which is cooled by pipes with sea water. This cooling water is heated by this and
can then be used in the distillation.
E.7 - Soil
The term soil organic matter (SOM) refers to all the organic matter in soil including living matter (active roots, living organisms)
and non-living components (root exudates, decomposing plant and animal material, humus and charcoal).
Humus refers to any organic matter that has reached a point of stability, where it will break down no further and might,
if conditions do not change, remain as it is for centuries, if not millennia. So it is the end-product of decomposition.
Humus should be differentiated from decomposing organic matter in that the latter is rough-looking material,
with the original plant remains still visible, whereas fully humified organic matter is uniform in appearance
(a dark, spongy, jelly-like substance) and amorphous in structure, and may remain such for millennia or more.
Soil is a complex mixture of inorganic and organic materials, including living organisms. Salinization
Salinization is the result of continual irrigation of soil. In poorly drained soil, after the water evaporates, salt is left behind, and plants
die because they are unable to take water away from the salty soil.
Nutrient depletion
Plants remove nutrients and minerals from soil as they grow. If not properly managed by crop rotation or fertilizing the soil,
nutrients will become depleted.
Soil pollution
Soil pollution is caused by improper use of pesticides and over-fertilizing. Chemicals can disrupt the food cycle, reducing the soil’s biodiversity,
and ultimately ruining the soil.
SOM refers to the organic constituents in the soil. This includes plant and animal tissue, partial decomposition products and soil biomass.
Chemicals found in SOM from decomposition of plants are high molecular mass organics such as Polysaccharides, proteins, sugars, and amino acids.
The end product of decomposition is humus. Humus is the organic decomposition layer which plants live on. It has a mixture of simple and more complex
organic chemicals from plants, animals, or microbial origin.Biological functions
1. It is a source of energy and nutrients (P, N, S).
Nitrogen provides proteins, Phosphorus provides enzymes,
Sulfur provides amino acids.Physical functions
1. It improves the soil structure.
2. Humus dark colour absorbs heat and keeps the soil warm.Chemical functions
1. Humus has the ability to maintain a constant pH by acting as a buffer because organic acids and their salts acts as a buffer in humus.
TILLAGE
Here is a list of common soil pollutants and their major sources:
E.12 - Water and Soil
Assume that you have a salt (MS) in a water solution so that it contains metal ions (M2+ = Hg2+ or Pb2+ or Zn2+ )
and non-metal ions such as sulfide ions (S2- ).2+ (aq) + S2- (aq)sp = [M2+ ] [S2- ]
A small Ksp means that the salt has a low solubility.
Ksp depends only on the temperature.
Assume you have heavy metal ions (Hg2+ or Pb2+ or Zn2+ ) in waste water and you want to remove them.2 S) to the solution you will create a metal salt (HgS):2+ (aq) + H2 S(aq) → HgS(s) + 2H+ (aq)2+ (aq) + S2- (aq)2- one puts in the more one will push the equilibrium to the left and the more salt is produced.
MS(s) ↔ M2+ (aq) + S2- (aq)sp = [M2+ ] [S2- ]2+ ] = Ksp / [S2- ]2- the concentration of
metal ions M2+ has to go down.
Salt in the water dissolves, however if some remains in solid form, it will eventually be in equilibrium. Remember these 5 steps:sp = [ion conc.][ion conc.])sp
if appropriate.)
Silica is another name for Silicon dioxide SO2 .
Clay consists of giant covalent Silica structures. However, a large part of the Silicon atoms in the structure are
replaced by Al3+ ions. This creates a surplus of electrons which attracts positive ions (Na+ , K+ ,
Ca2+ , Mg2+ ) to its surface.
There are two parts of the soil that can exchange cations (positive ions) with other cations that are in the water of the soil:CLAY: The cations that are on the surface of the clay are not held tightly and so they can be exchanged. Example:+ (clay) + H+ (aq) ↔ H+ (clay) + K+ (aq) HUMUS: In humus (SOMs) there is weak organic acids and their salts which makes cation exchange possible. Example:+ (aq) ↔ RCOOH(humus) + Na+ (aq)
Cation-exchange capacity (CEC):
Both SOM and clay have negatively charged particles which will bond to cations such as Ca+2 , Mg+2 , Na+ , K+ ,
Al+3 and
the amount of positively charged cations that soil can hold is called the cation-exchange capacity (CEC).
A larger CEC indicates a larger capacity to hold cations. These cations are exchanged with cations such as hydrogen on the root hairs of a
plant to provide it with nutrients. CEC is defined as
the number of moles of singly charged positive ions that can be held in 1 kg of soil.Importance: A large CEC value means the soil can absorb many cations and make them available to plants so a high CEC value means more nutrients
for the plants.
The growth of plants depends on the amount of nutrients which in turn depends on the pH. The pH of soil varies between
3 to 9 but a pH between 6 and 6.5 (slightly acidic) gives the best availability of most nutrients.
E.8 - Waste
Method of disposal | Advantages (+) | Disadvantages (-)
There are 3 main benefits to recycling that apply to metal, glass, plastic and paper. These are:
Low-level waste includes any gloves, paper towels or protective clothing that has been used in areas where radioactive materials have been handled.
The level of activity is low and the half lives are short. This waste generally comes from hospitals due to cancer treatment, and includes any items that have come in
contact with the radioactive material.
The nuclear decay process produces heat and energy. Low-level waste is stored in cooling ponds until the activity has fallen to safe levels (generally a few years).
The water is then passed through ion exchange resins which remove isotopes responsible for activity. The water is then diluted and released into the sea.
Terminology
aerosols .