ANSWER
Download notes on bonding
Go back to the IB chemistry page
Go to the IB physics page
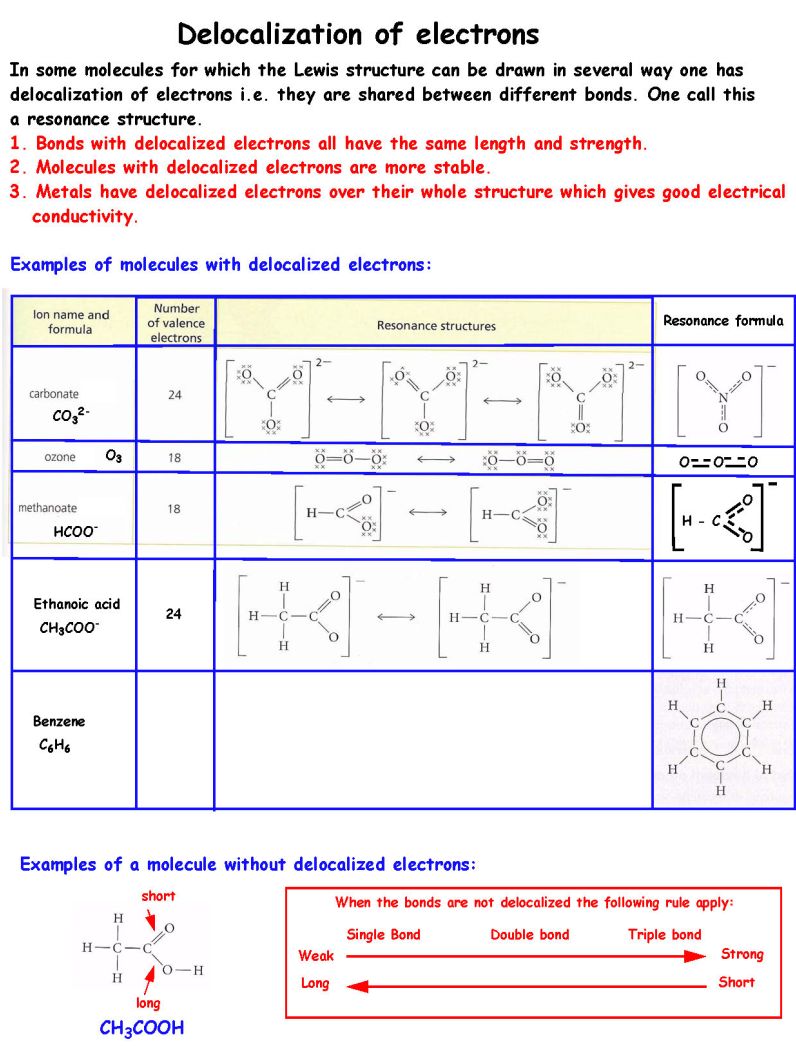
Correct answer is C because:

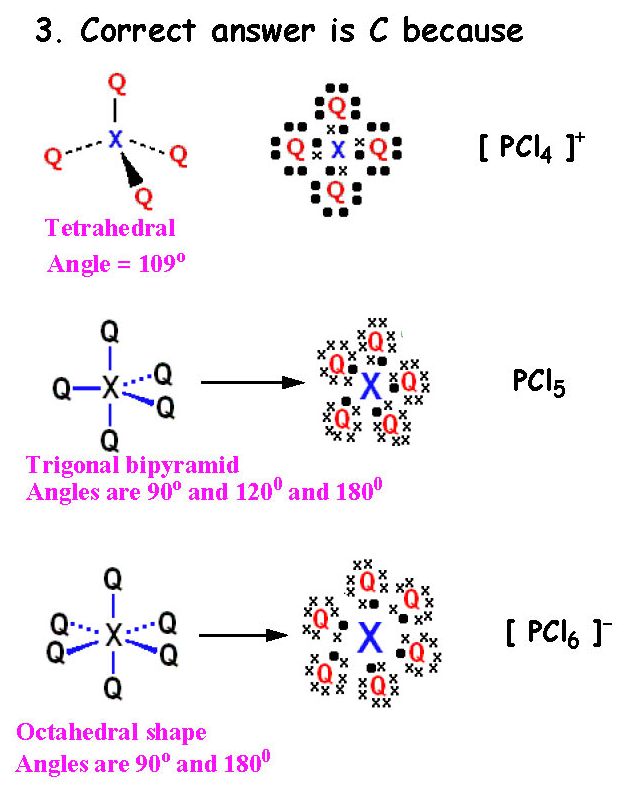
Correct answer is C because:
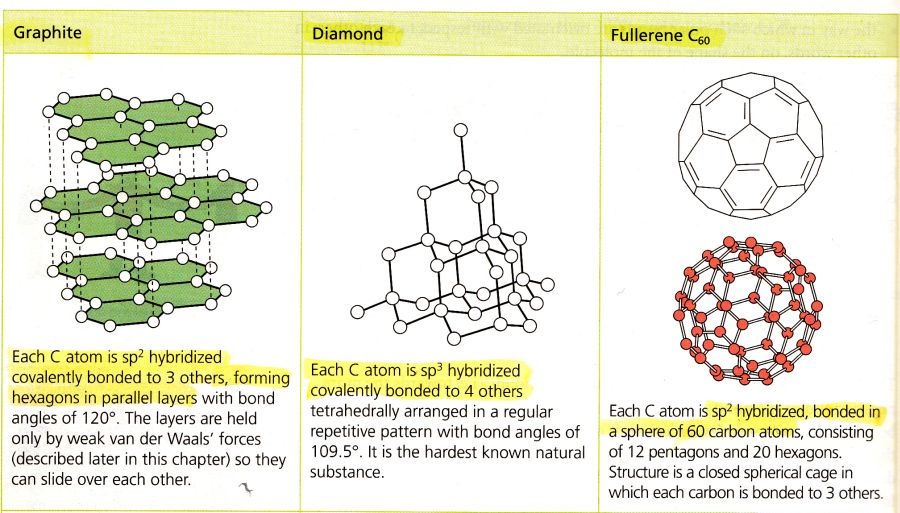
Correct answer is C because:
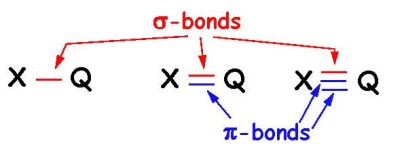
Sigma bonds are formed by either s or p orbitals
Pi bonds are formed only by p orbitals
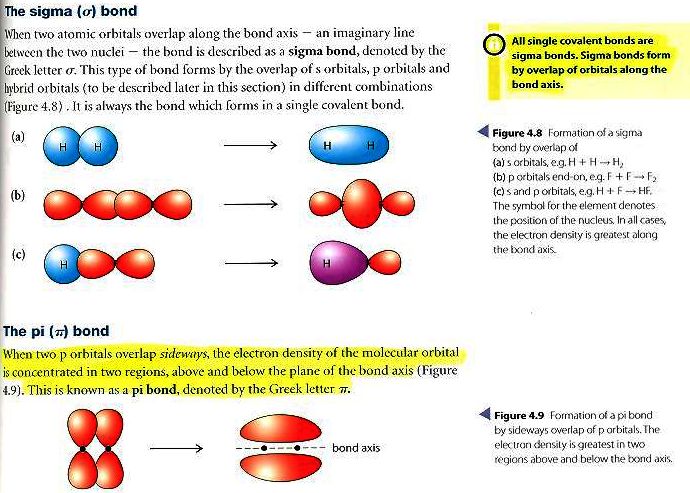
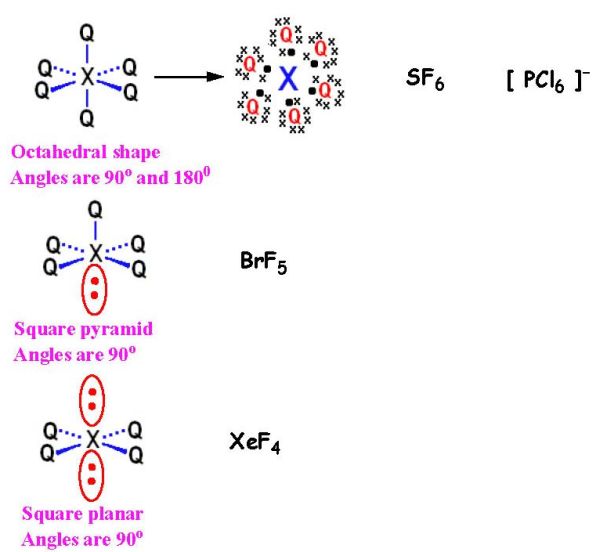
Correct answer is B because:


Correct answer is B because:
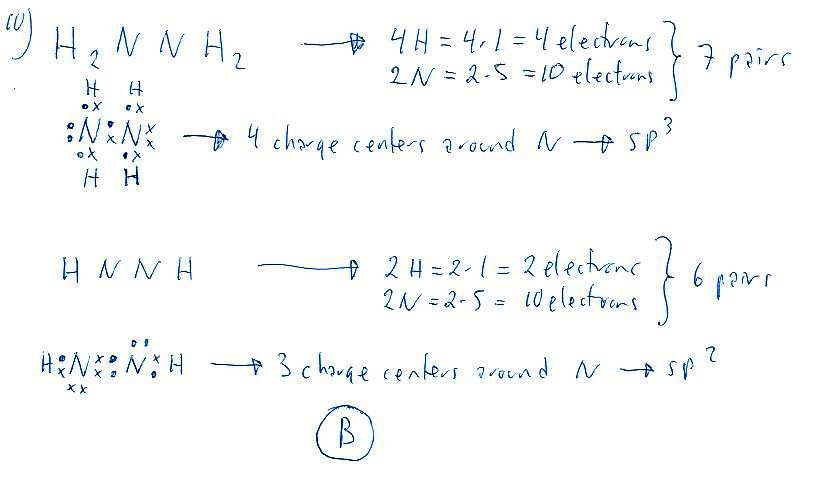
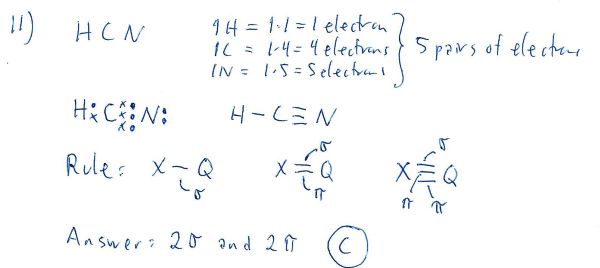
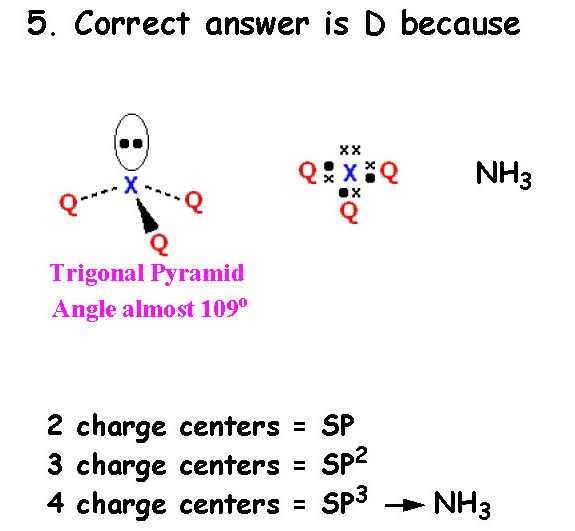
Correct answer is D because:

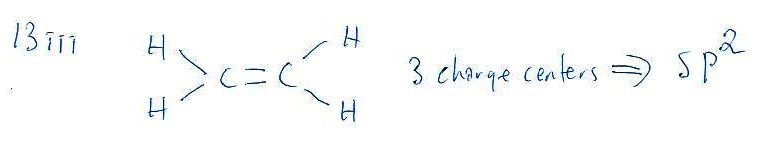
3 charge centers means SP2 hybridization
Hybridization means mixing or merging of orbitals.
2 charge centers means SP hybridization
A triple bond always has one sigma and two pi bonds