
1 - What is the value of Planck's constant ?
3 - What is the velocity of a photon ?
4 - How can one calculate the wavelength of a particle if one knows its frequency ?
5 - What is the formula for the energy of a photon ?
6 - What is the formula for the momentum of a photon ?
7 - How does the energy of light depend on the intensity of the light ?
8 - What is relativistic mass ?
9 - What is mass of a photon ?
10 - What is Einstein's famous formula and what does it mean ?
11 - What is the photoelectric effect ? What is its formula ? Draw curve !
12 - Draw a picture and explain the zinc plate experiment.
13 - Describe Millikans photo electric experiment.
14 - Explain why atoms can only send out photons with certain fixed energy (or frequency) ?
16 - Explain how an electron gun works.
17 - What is the de Broglie hypothesis ?
18 - What is the formula for the momentum of an electron according to de Broglie hypothesis ?
20 - An electron is accelerated with a potential that is 1000 V, what is the energy in Joule ?
21 - What sort of experiments can be done to verify the de Broglie hypothesis ?
22 - What is Heisenberg's uncertainty principle ?
24 - Explain the "electrons in a box" model of an atom.
25 - Explain the Schrödinger model of an atom.
27 -
Fill in this table: 
28 -
What states can the electron in an atom be in according to the Schrödinger model ?
Look at the data booklet: 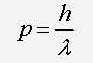
Since c = f λ
and E = h f
this means that
E = p c