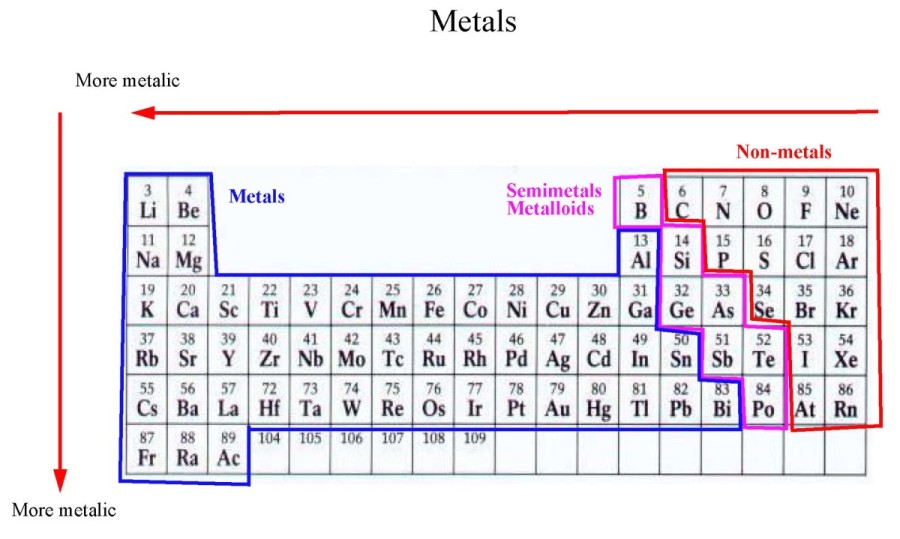
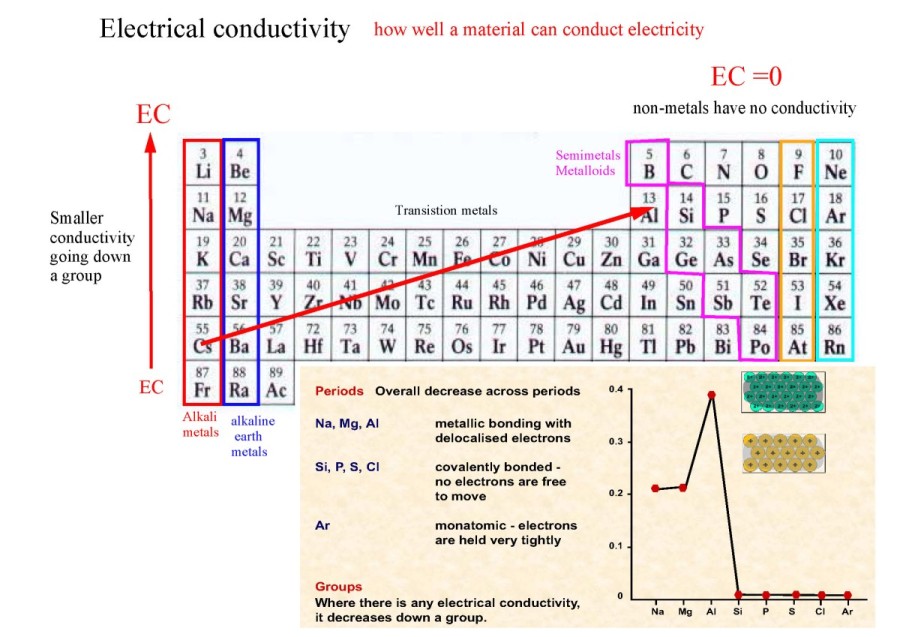
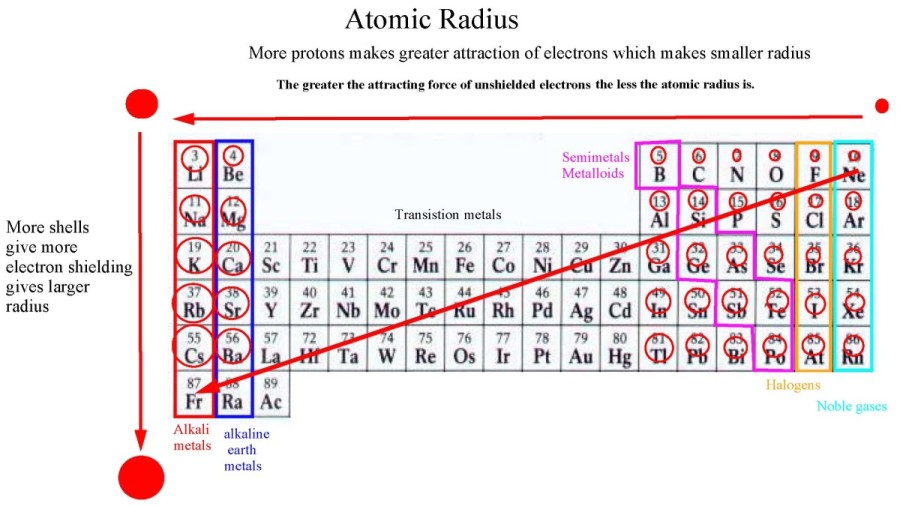
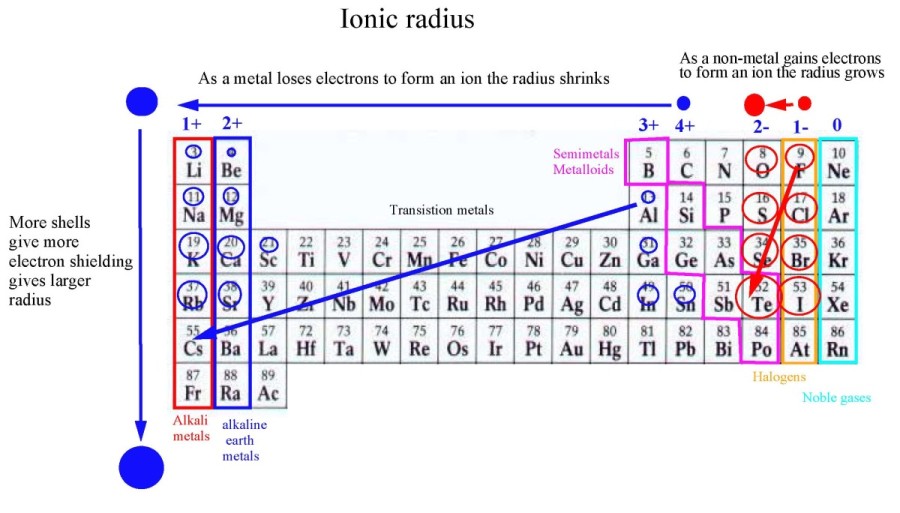
Elements increase in atomic number across each period, and down each
group.
Group – the columns going down = number of valence electrons in the atom.
Period – the rows going across = number of
main electron shells…s, p , d and f blocks.
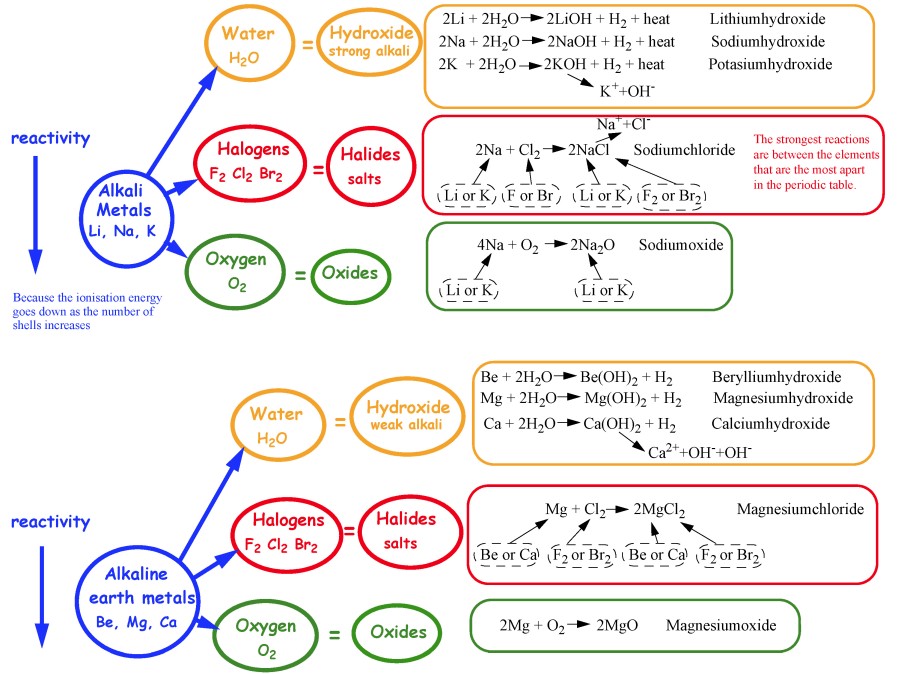
Reactions of elements in the same group are similar because they have
identical outer shells (ie same number of valence electrons).
Alkaline earth metals:Be, Mg, Ca....Be2+ Mg2+, Ca2+....
Halogens:F2, Cl2, Br2, I2.....
F-, Cl-, Br-, I-.......
Noble gases: Ne, Ar, Kr, Xe ..... no ions
Alkali metals:
Li, Na, K....Li+, Na+, K+.... Nuclear charge = Z = the number of protons. Atomic and ionic radius increases due to increased electron shielding.
The outer electron is in an energy level that is progressively further
away from the center of the nucleus.
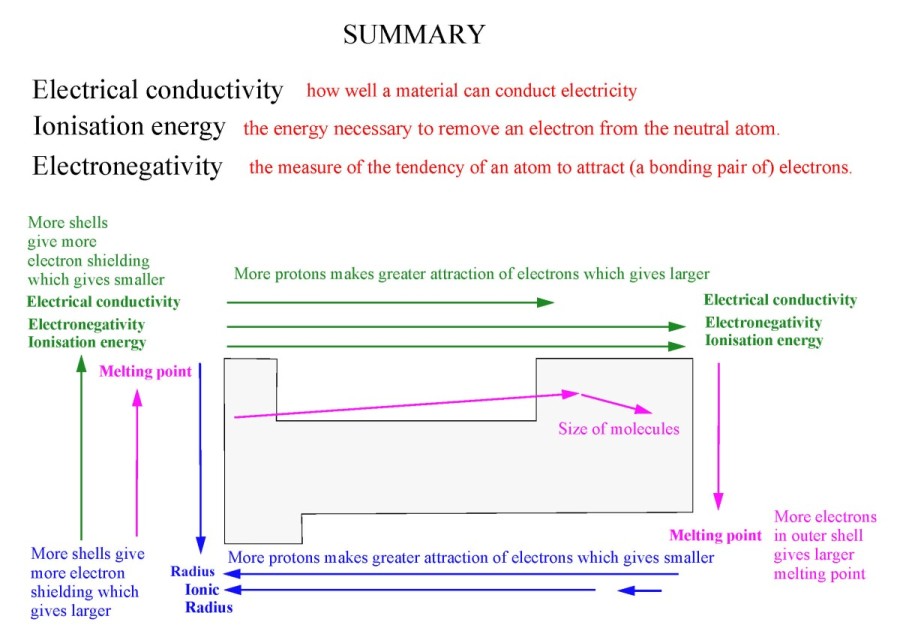
Ionisation energy decreases due to increased electron shielding.
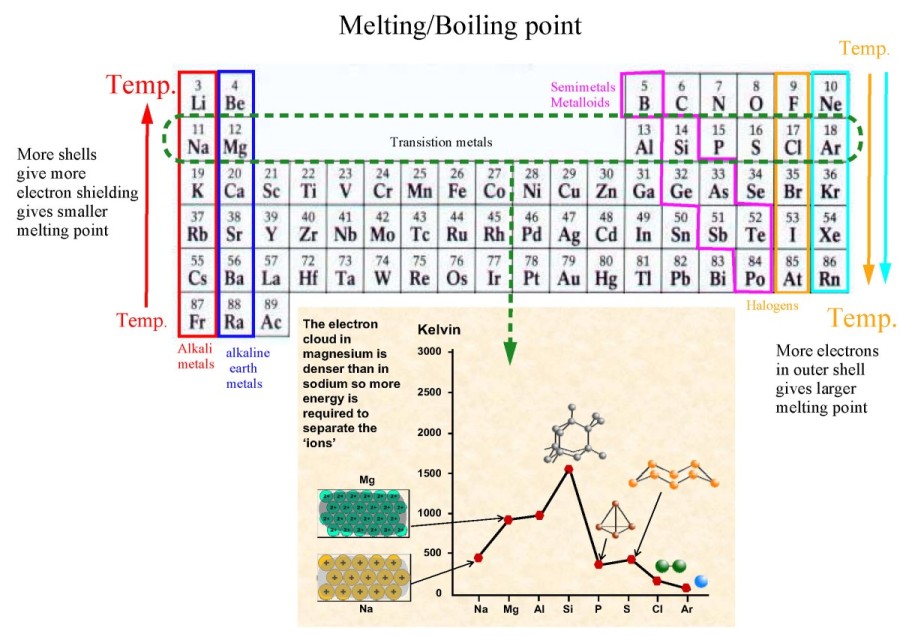
Melting/boiling point decreases due to increased electron shielding (hence decreased forces).
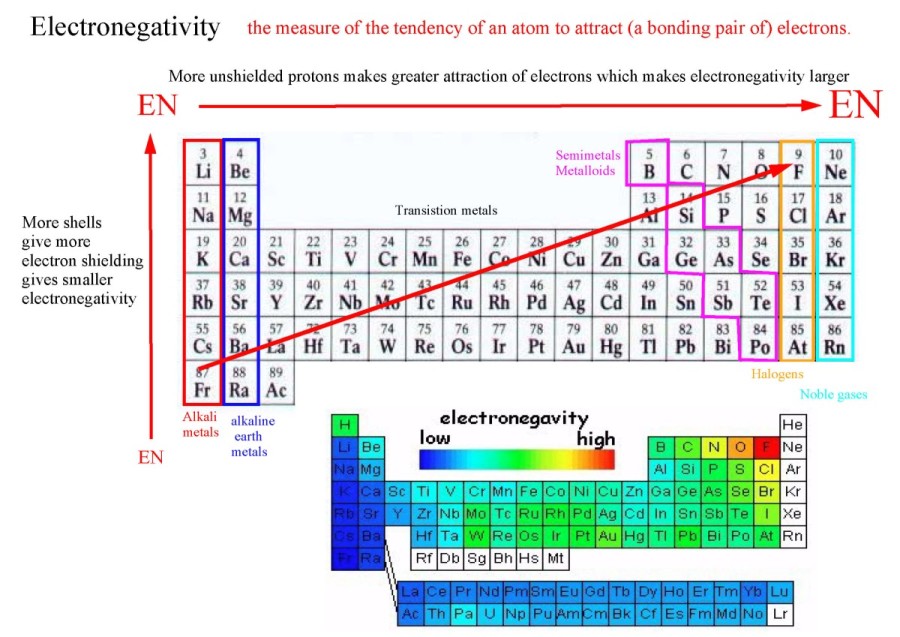
Electronegativity decreases due to increased shielding (hence decreased attraction for outer electrons).
Atomic and ionic radius increases due to increased electron shielding.
Ionisation energy decreases due to increased electron shielding.
Melting/boiling point increases due to increased number of electrons->increased london dispersion forces.
Electronegativity decreases due to increased shielding -> decreased attraction for outer electrons.
Atomic radius decreases due to increased nuclear charge -> greater attraction for electrons.
Ionic radius decreases Na->Al (due to increased nuclear
charge) jumps Al->Si (due to reversal of ionisation
direction…increased electron-electron repulsion) decreases Si->Ar
(due to increased nuclear charge).
Ionisation energy increases due to increased nuclear charge.
Melting/boiling point increases Na->Si (due to stronger
metallic bonding – more delocalized electrons then network covalent)
drops Si-P (due to network->molecular covalent) increases P->S
(due to increased LDF between molecules i.e. P4, S8). Drops to Cl, due to smaller
molecules (Cl2) decreases to Ar (individual atoms->fewer electrons->smaller LDF).
Electronegativity increases due to increased nuclear charge -> greater attraction for electrons.
The electrons in the inner shells shield the electrons in the outer shells so the effective
charge that these electrons experience is less than Z.
Li->Cs (down the alkali metals)
F->I (Down the halogens)
Na->Ar (across period 3)