2. What is the electron arrangement and electron configuration for oxygen ?
3. Draw a sketch showing the number and names of shells, sub-shells and orbitals.
4. What does the s and p orbitals look like ?
5. What does the d orbitals look like ?
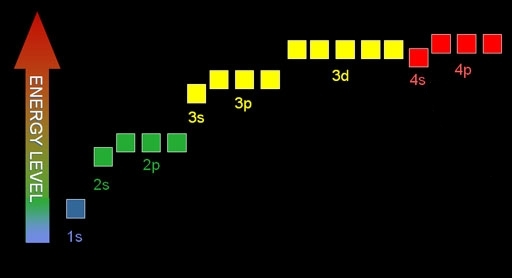
6. What are the energy levels of the different orbitals ?
7. How many electrons can you have in the shells, sub-shells and orbitals ?
8. What is the Pauli Principle ?
10. What is the Aufbau principle ?
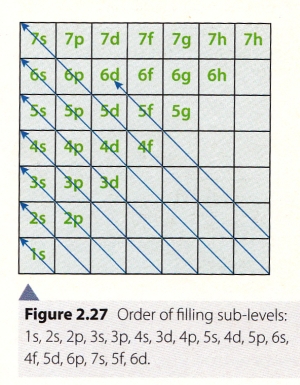
11. How can one figure out in which order levels are filled ?
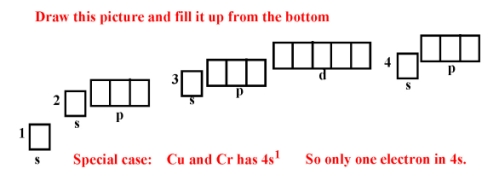
12. How can one figure out the electron configuration ?
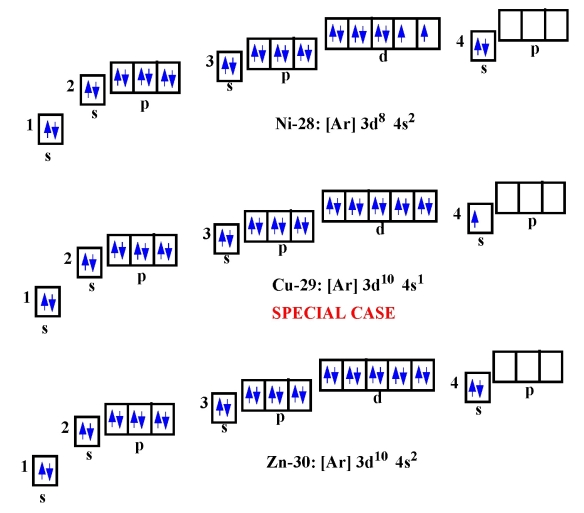
13. Get electron configurations for Ni-28, Cu-29 and Zn-30 !
14. Get electron configurations for K-19, Vr-23 and Cr-24 !
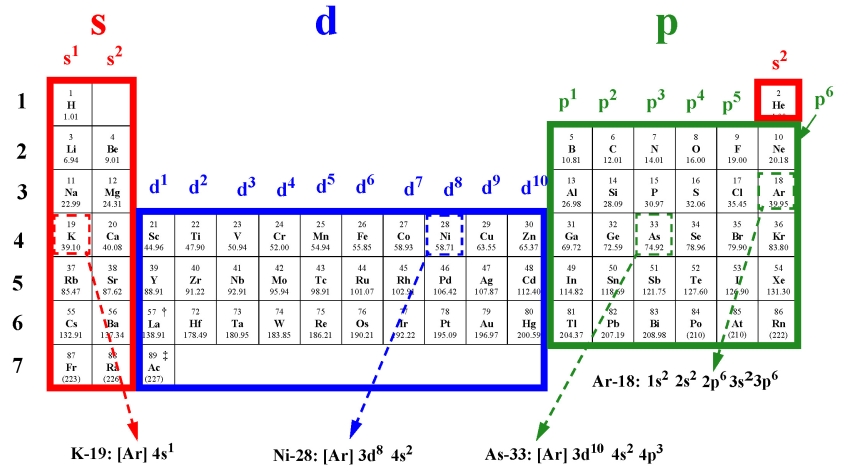
15. Use the periodic table to get electron configurations for Ar-18, K-19, Ni-28 and As-33 !
16. Use the periodic table to write down the electron configuration for Iodine.
17. Draw the shape of the s and p orbitals.
18. How can ionisation be used to show the electron configuration of atoms ?
Download notes on electron shells.
Go back to the IB chemistry page
Go to the IB physics page
Electron arrangement: The number of electrons in each main shell (n).
Electron configuration: The number of electrons in each sub shell (s, p, d. ...).
Electron arrangement: 2, 6
Electron configuration: 1s22s22p4
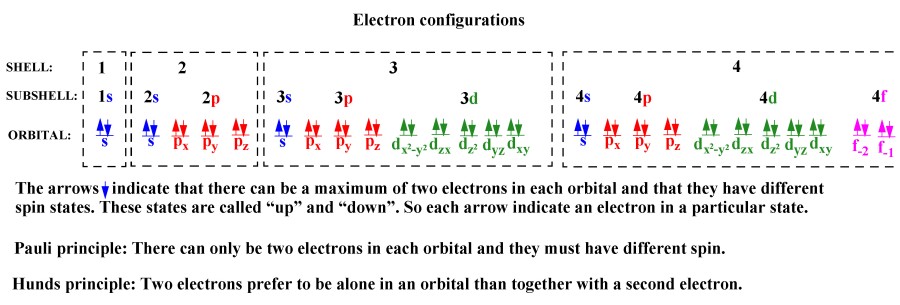
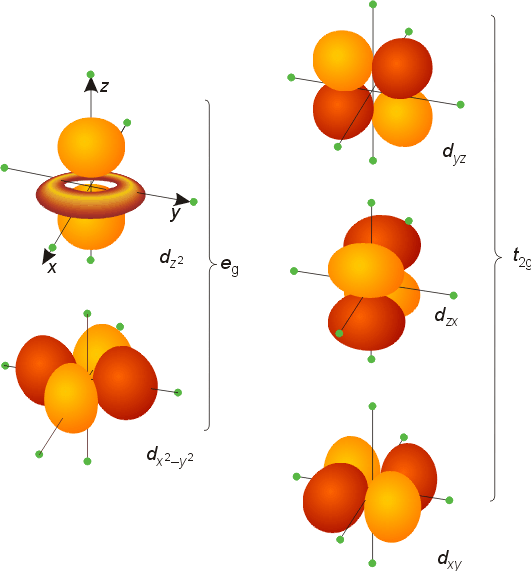
Number of electrons in a shell: 2, 8, 18, .....2n2 for n = 1 , 2, 3 .......
Number of orbitals in a sub-shell: s=1, p=3 d=5 f=7......
Number of electrons in an orbital: always max 2 electrons in each orbital. One with spin up and one with spin down.
Number of electrons in a sub-shell: s=1x2=2, p=3x2=6 d=5x2=10 f=7x20=14......
Pauli Principle: Only two electrons can be in one orbital and they must have different spin. (They must have different quantum numbers).
Hund's rule: If more than one orbital in a sub-level is available then the electrons will be in different orbitals with different spin. Because this minimize the energy.
Aufbau principle: Electrons are placed into the orbitals of lowest energy first.

The electron configuration for Iodine is [Kr]5s24d105p5.
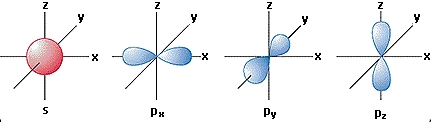
Shapes of orbitals :
s orbital is a sphere around the nucleus.
p orbitals are shaped like a figure 8 (and there are 3 of them on
perpendicular x, y and z axes around the nucleus).
Successive electrons can be stripped from an atom until there is only
the nucleus left. If the energy required to achieve this for each
electron is plotted on a graph (with a log scale) against ionisation
number, the 'jumps' in the required energy clearly show the main and sub
energy levels.