Exothermic reaction: A reaction which produces heat. ΔH = negative
Endothermic reaction: A reaction which absorbs heat. ΔH = positive
So if the reaction produces heat (increases the temperature of the surroundings) then it is exothermic. If it decreases the temperature (i.e. absorbs heat) then it is endothermic. Also, the yield of an equilibrium reaction which is exothermic will be increased if it occurs at low temps, and for endothermic reactions the yield increases at high temperatures.
Energy is the ability to do work. Unit: Joule.
Temperature is a measure of the kinetic energy of the molecules.
Heat is a form of energy which is transferred as a result of a temperature difference.
Open system: Can exchange energy and matter with the surroundings e.q. an open bottle.
Enthalpy (H) is the heat content of a substance. The enthalpy of a reaction is the change in internal energy through a reaction and
is called ΔH and it is negative for exothermic reactions (because internal heat is being lost) and positive for endothermic reactions (because internal energy is being gained).
The Standard Enthalpy Change (ΔHo) is the change of H for a set of standard conditions:
Most reaction are exothermic (they give off heat - the reactants have the highest energy i.e. temperature). Examples: all combustion and neutralization reactions.
1 J is the work done when a body is moved 1 m against a force of 1 N.
Closed system: Can only exchange energy with the surroundings e.g. a closed bottle.
1. The temperature is 25oC.
2. The pressure is 100 kPa.
3. The concentration is 1 mol/dm3 for all solutions.
4. All substances are in their standard states (gas, liquid or solid).
A few reaction are endothermic (they take up heat - the products have the highest energy i.e. temperature). Examples: photosynthesis.
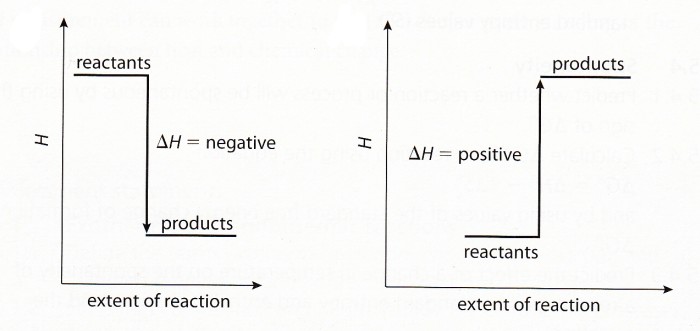
The most stable state is where all energy has been released. Therefore when going to a more stable state, energy will be released, and when going to a less stable state, energy will be gained. On an enthalpy level diagram, higher positions will be less stable (with more internal energy) therefore, if the product is lower, heat is released (more stable, ΔH is negative) but if it is higher, heat is gained (less stable, ΔH is positive).