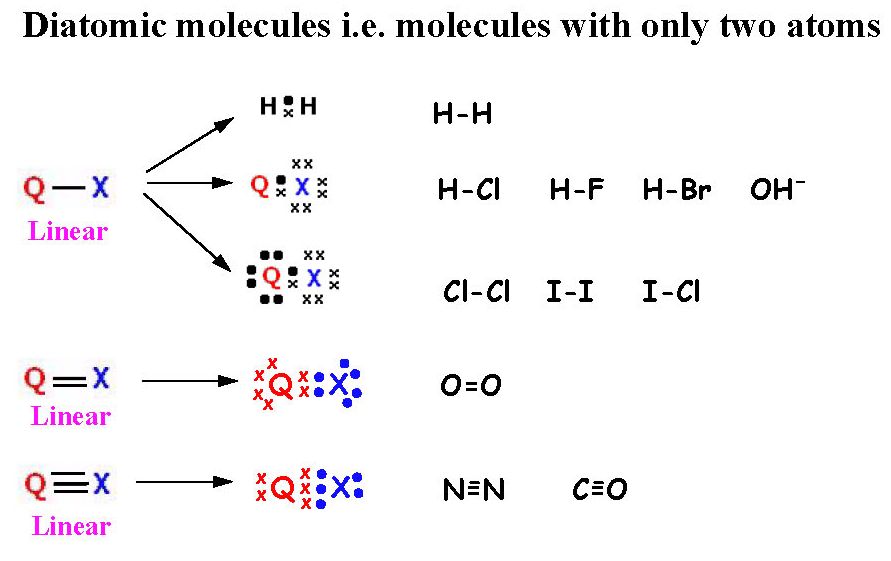
1 - Explain the shape of diatomic molecules
2 - Explain the shape and hybridization of molecules with two charge centers
3 - Explain the shape and hybridization of molecules with three charge centers
4 - Explain the shape and hybridization of molecules with four charge centers
5 - Explain the shape and hybridization of molecules with five charge centers
6 - Explain the shape and hybridization of molecules with six charge centers
H3O+
NH4+
PCl6-
OH-
PCl4+
PF3-
SO42-
Download notes on bonding
Go back to the IB chemistry page
Go to the IB physics page