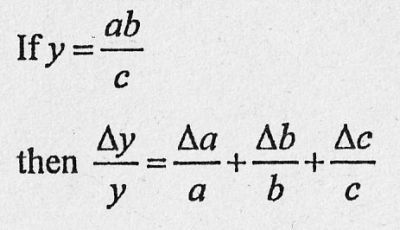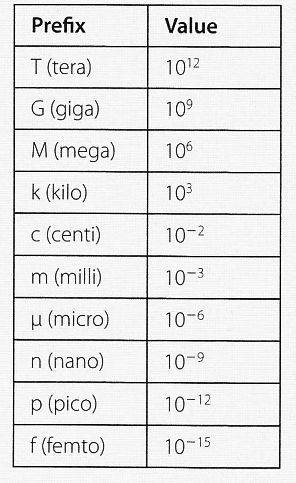
1 - What do the prefixes nano, giga, micro, mega, kilo, pico and milli mean ?
2 - Convert 12.3 liters to m3 and write it in the standard form !
3 - Which are the fundamental units that all other derived units are made from ?
4 - What is the formula for density and what unit does it have ?
5 - What is the density of water and air ?
6 - How do you estimate the error on a scale on for example a ruler ?
7 - Explain the difference between accuracy and precision and random error and systematic error.
8 - You measure 124.123456±0.0234546 . How should you report this value ?
9 - You measure 124.123456±2.34546 . What is the absolute and the relative error ?
9.4 -
You measure a=1.1±0.1 b=2.1±0.3 b=3.1±0.3
calculate y = a + b - c
9.5 -
You measure a=1.1±0.1 b=2.1±0.3 b=3.1±0.3
calculate y = ab/c
10 - Explain how you can estimate the error on the gradient of a straight line measurement !
11 -
What is a scalar and vector quantity ?
Density: ρ = m / V where m is the mass and V is the volume. Unit: kg/m3
Density of water = 1.0 x 103 kg/m3
Density of air = 1.2 kg/m3
If you measure 124.123456±0.0234546 you should report it as 124.12±0.02
If you measure two values a and b with the errors Δa and Δb
and you calculate a new quantity y = a + b.
Then you find the error on y in the data booklet:
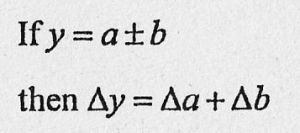
If you measure two values a and b with the errors Δa and Δb
and you calculate a new quantity y = a - b.
Then you find the error on y in the data booklet:
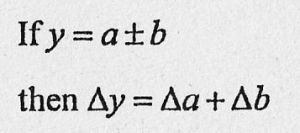
If you measure three values a, b and c with the errors Δa , Δb and Δc
and you calculate a new quantity y = ab/c .
Then you find the error on y in the data booklet: