HOW TO GET JAVA TO RUN THE JAVA FILES ON THIS PAGE.
8.1 - Energy degradation and power generation - questions
1 -
How can one make energy ?
2 -
What is a fuel ?
3 -
What does the second law of thermodynamics tells us ?
4 -
What is degradation of energy ?
5 -
What is the energy transfer that takes place in the following cases ?
a) Light bulb ?
b) Electric motor ?
c) Battery ?
d) Car engine ?
e) Solar cell ?
6 -
Explain how an electric generator works.
7 -
Explain how an electric motor works.
8 -
Draw a Sankey diagram of a car engine and explain where the energy is degraded.
9 -
Draw a Sankey diagram of an electric motor and explain where the energy is degraded.
8.2 - World energy sources - questions
10 -
What are fossil fuels ?
11 -
What are three main things fuels are used for ?
12 -
What are renewable and non-renewable energy sources ?
13 -
What are the most used sources of energy in the world ?
14 -
What are the most used sources of electrical energy in the world ?
15 -
What is energy density and what fuel has the largest energy density ?
16 -
Make a list of energy sources and note which are renewable !
17 -
Which ones are fuels ?
18 -
Which ones are used for electricity production ?
19 -
What are the problems with the different sources ?
8.3 - Fossil fuel power production - questions
20 -
How does a coal power station work ?
21 -
How does a gas power station work ?
22 -
Compare the energy density of wood to that of fossil fuels and explain why they are better.
23a -
What are the advantages of fossil fuel ?
23b -
What are the disadvantages of fossil fuel ?
23c -
Compare different fossil fuels.
8.4 - Nuclear power - questions
24 -
Explain what fission is ?
25 -
Explain what a chain reaction is.
26 -
What is a moderator ?
27 -
What are the control rods ?
28 -
What is nuclear fuel made of ?
29 -
How does a nuclear power station work ?
30 -
How does an atomic bomb work ?
31 -
Explain how plutonium is produced in a nuclear reactor.
32 -
What are the problems with nuclear energy ?
33 -
What are the good things about nuclear power ?
34 -
What are the difficulties in building a fusion reactor ?
8.5 - Solar power - questions
35 -
How does a solar panel work ?
36 -
How does a photovoltaic cell work ?
37 -
What are the advantages of solar energy ?
38 -
What are the disadvantages of solar energy ?
39 -
What is the intensity ?
40 -
What is the albedo ?
8.6 - Hydroelectric power - questions
41 -
How does a hydroelectric power station work ?
42 -
What are the advantages of hydroelectric power ?
43 -
What are the disadvantages of hydroelectric power ?
44 -
How can one calculate the power of a hydroelectric station ?
8.7 - Wind power - questions
45 -
How does a wind power station work ?
46 -
What are the advantages of wind power ?
47 -
What are the disadvantages of wind power ?
48 -
How can one calculate the power of a wind power station ?
8.8 - Wave power - questions
49 -
How does a wave power station work ?
50 -
What are the advantages of wave power ?
51 -
What are the disadvantages of wave power ?
52 -
How can one calculate the power of a wave ?
53 -
How can one calculate the speed of a wave ?
8.9 - Green house effect - questions
54 -
The sun radiates Isun=3.9x1026 W.
How can one calculate the intensity of the sunlight on a planet at a distance d ?
55 -
Explain the difference between light interacting with gases and solids.
56 -
Explain what black body radiation is.
57 -
Draw the back body spectrum for a couple of different T.
58 -
What is Stefan-Boltzmanns law ?
59 -
Draw a diagram of the Stefan-Boltzmann law.
60 -
What is emmisivity ?
61 -
What is the greenhouse effect ?
62 -
What is the surface heat capacity ?
63 -
Explain with a formula how the temperature will change with the intensity.
8.10 - Global warming - questions
64 -
Give three examples of reasons for global warming that are not caused by humans.
65 -
What is the enhanced greenhouse effect ?
66 -
Which are the most important greenhouse gases ?
67 -
What evidence is there that CO2 causes global warming ?
68 -
What is the likely cause of the increase of the CO2 levels in the atmosphere ?
8.11 - What might happen - questions
69 -
What is the coefficient of volume expansion ?
70 -
What problems will global warming cause ?
71 -
How can global warming be reduced or stopped ?
8.1 - Energy degradation and power generation
Energy cannot be made. It is conserved. But it can be changed from one form to another.
A fuel is a substance that can release energy by changing its chemical or nuclear structure.
When something is burning the positions of the atoms in the fuel is changing so that the potential
energy of the atoms becomes lower. This is compensated for by an increase of the kinetic energy of the
atoms i.e. heat is produced.
The second law of thermodynamics tells us that one
cannot turn thermal energy into work without loosing some of the thermal energy to the surroundings.
The typical example is an engine or a motor. It is always true that in an engine some of the chemical energy
in the fuel will be lost because things will heat up. The efficiency of an engine can never be exactly 100%.
Degradation of energy is the
transformation of energy into some form in which it is less available for doing work. Thermal energy is the most degraded form of energy.
Examples of energy transfer
a) Light bulb: Electrical energy → Light + Heat
b) Electric motor: Electrical energy → Mechanical energy + Heat
c) Battery: Chemical energy → Electrical energy + Heat
d) Car engine: Chemical energy → Mechanical energy + Heat
e) Solar cell: Light → Electrical energy + Heat
The electric generator
One puts a coil in a magnetic field (blue arrows).
One turn the coil around by letting for example air, water or steam hit a propeller (orange handle).
A current will then be generated in the coil that goes backwards and forwards (red arrows).
The alternating current will generate an alternating voltage as shown in the diagram.
The electric motor
One puts a coil in a magnetic field (blue arrows).
One sends a current through the coil (red arrows).
There will then be a force on the coil (black arrows) that will make the coil turn.
The turning coil can then be connected to an axis that turns for example the wheels of
an electric car.
Sankey diagram of a car engine
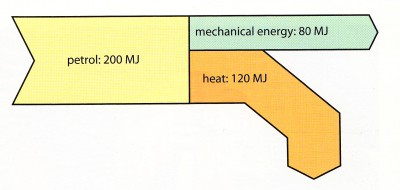
(You do not have to learn the numbers in the plot)
The degradation happens when 120 MJ of energy is lost as heat instead of being turned into
mechanical energy.
Sankey diagram of an electric motor
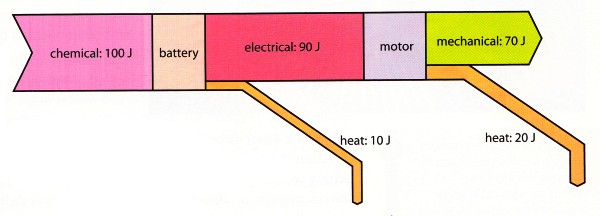
(You do not have to learn the numbers in the plot)
The degradation happens firstly when 10 J of energy is lost as heat when the battery
is heating up while producing electrical energy.
And secondly when 20 J is lost as the
motor is heating up while producing mechanical energy.
8.2 - World energy sources
Coal, oil and gas are called "fossil fuels" because
they have been formed from the organic remains of
prehistoric plants and animals.
Fuels are used for:
1) Generate electricity
2) Heat houses
3) Power engines (car, boat and airplane engines etc).
Oil and gas is used for all three. Coal is used for 1)
and sometimes 2) but not 3).
Non-renewable energy sources are most fuels (oil, gas, coal, nuclear) that will eventually run out.
Renewable energy sources are things that will not run out: Hydropower (water dams), wind, solar, geothermal but also fuels made of wood and other plants (biofuel).
If you look at all forms of energy use (electricity, heating, engines) then the fossil
fuels (oil, coal and gas) are the most used sources of energy. After that comes nuclear and hydropower
with a smaller contribution. Other stuff (solar, wind, geothermal, wood) is used very little.
If one only looks at the sources used for electric energy production then in order of importance comes:
coal, gas, nuclear and hydropower. Oil is used very little.
Energy density is the amount of energy that can be extracted from each kg of fuel. Nuclear
fuel has an energy density that is several million times larger than other fuels.

8.3 - Fossil fuel power production
Coal power station
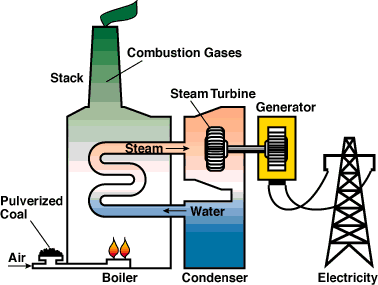
Coal is burning and makes water boil. The steam is used to turn
a turbine that is connected to a generator.
The steam is then cooled
down back to water in a condenser.
For more detail click on this
link.
Gas power station
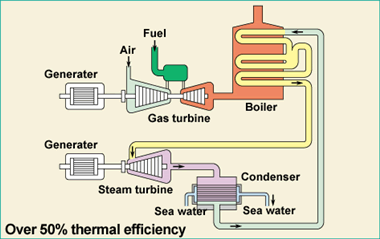
Natural gas is sent into a gas turbine where it is ignited. The gas turbine turns a generator.
The hot gases from the gas turbine are used to boil water and the steam is used to turn a second turbine + generator.
For more detail click on this
link.
Energy density
Wood: 17 MJ/kg
Coal: 33 MJ/kg
Petrol: 47 MJ/kg
Liquid natural gas: 54 MJ/kg
So there is double as much energy in coal as in wood and there is 3 times more energy in gas than in wood.
This explains why coal and gas is mainly used to produce electricity and not wood.
Advantages of fossil fuel
1. Relatively cheap (while they last).
2. High energy density.
3. Easy to use with engines and devises.
4. Distribution network in place.
Disadvantages of fossil fuel
1. They will run out.
2. They pollute the environment.
3. They produce greenhouse gases.
Comparing different fossil fuels
Oil is easier to transport than coal and gas because it is a liquid.
However, if an oil tanker sinks there is an environmental disaster.
Coal is a dirtier fuel than oil and gas.
Oil is easier to get out of the ground than coal.
8.4 - Nuclear power
Fission
Fission is when very large nuclei is split into two smaller nuclei + a couple of neutrons. This is normally
done by hitting the large nuclei with slow neutrons.
In the plot below a U-235 atom is split into one Kr-91 and one Ba-142 atom + 3 neutrons. The neutrons goes on
and split other U-235 atoms. One has a chain reaction. Every time a U-235 atom is split a small amount of mass
is turned into energy.
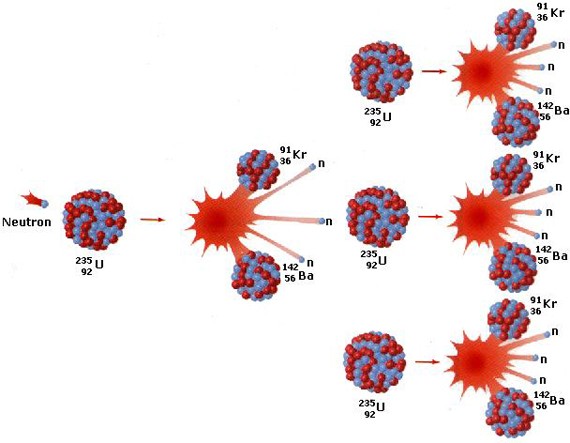
Chain reaction
Click on the plot to introduce a neutron among the green uranium atoms.
The red dots are the fission products (two are created from every uranium atom).
Moderator
Only slow neutrons will split the uranium atoms. In order to slow the neutrons down one puts
the uranium in a moderator (typically water) so that the neutrons collides with the water
and slows down before hitting the uranium.
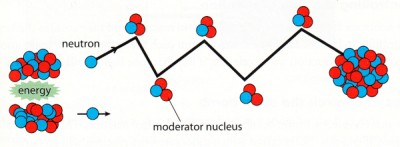
Control rods
One can slow down or stop the chain reaction by pushing in rods made of material that absorbs
neutrons (Boron or Cadmium) in between the uranium fuel. If one pulls out the rods
the chain reaction will increase. These rods are called control rods.
Nuclear fuel
Nuclear fuel is made of Uranium. Natural uranium that is coming out of the ground
consist to 99.3% of U-238 and 0.7% of U-235. Only U-235 has a large probability
to undergo fission when hit by a neutron. Therefore the percentage of U-235 has to be
increased from 0.7% to 3% before it can be used as a nuclear fuel. This process is called
enrichment and is a very difficult process. The enriched uranium is made into rods.
Between these rods one puts the moderator (water) and the control rods.
Nuclear reactor
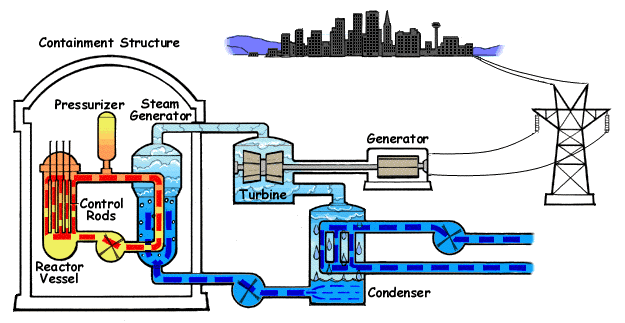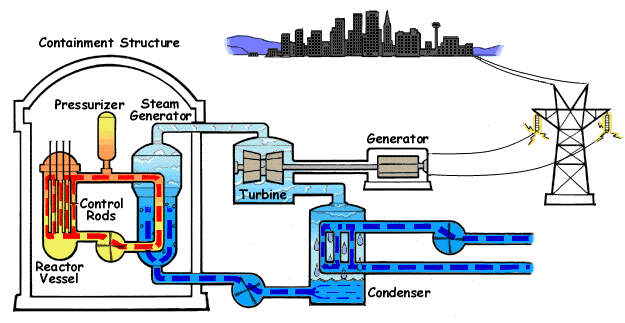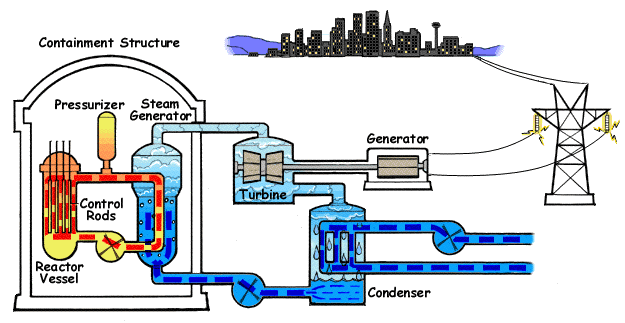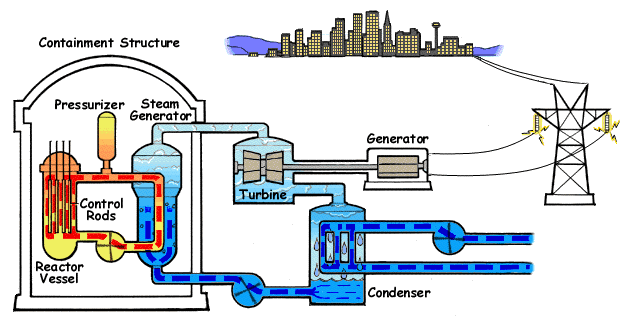
The chain reaction in the reactor vessel makes water boil.
The chain reaction is controlled with control rods.
This hot water goes to a steam generator where steam is generated.
The steam goes to a steam turbine that turns a generator.
The steam is then cooled down back to water in a condenser.
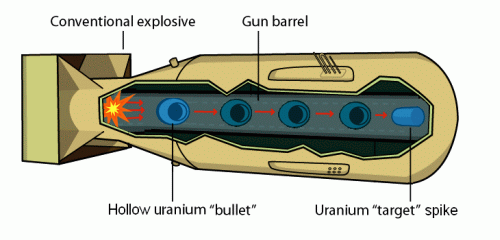
Atomic bomb
If one enrich the uranium so that one get more than 20% of U-235 one can get
an out-of-control chain reaction that leads to an explosion. The mass of Uranium needed
for an explosion is called the critical mass. If the mass is smaller, too many neutrons
escape through the surface. In a bomb one quickly put smaller uranium masses
together to get a critical mass when one wants the explosion to happen. One way of doing
this is by shooting a hollow uranium sphere onto a uranium spike.
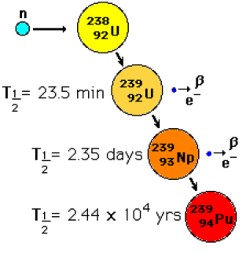
Plutonium
When the U-235 isotopes are hit by a neutron they split into two fragments and neutrons (fission).
When the U-238 isotopes are hit by a neutron they capture it and turn into U-239. The U-239 decay
to Np-239 which in turn decays to Pu-239.
When Pu-239 isotopes are hit by neutrons they can split like U-235 (fission). So they can also be used as
nuclear fuel (or in bombs).
Disadvantages of nuclear power
1. Mining of Uranium destroys the environment or gives radiation to mine workers.
2. If the cooling of the reactor stops the fuel gets very hot and melts. This meltdown can lead to a release
of very large amounts of radioactivity (but no nuclear explosion).
3. The fission process creates very radioactive waste that has to be stored for thousands of years.
4. The plutonium created in the reactors can be used to make atomic bombs.
Advantages of nuclear power
1. Very large power output (energy density).
2. Fission do not produce CO2 or any other greenhouse gases.
3. If one uses the plutonium that is created one can produce electricity for a very long time before all uranium is used up.
Problems with fusion power
One has to create and store a plasma that has a temperature of 100 million degrees. Another problem is to keep
the plasma hot while new fuel is added.
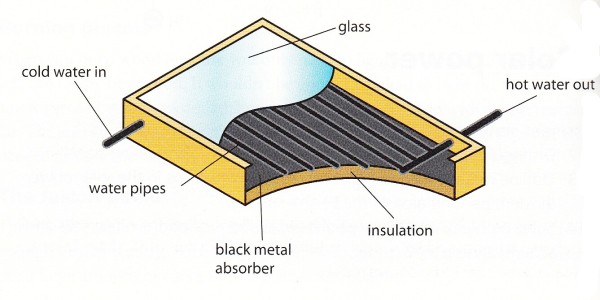
8.5 - Solar power
The solar heating panel is used to produce warm water. Water pipes are put between a glass window and a black metal absorber.
The sun light goes through the window (which traps it) and heat up the metal plate that heats up the water pipes.
The photovoltaic solar cell creates electrical energy. It consists of different types of semiconductors that creates
an electric field with free electrons.
Advantages of solar power
1. No cost of fuel.
2. Will never be exhausted.
3. Clean.
Disadvantages of solar power
1. Works only during the day.
2. Gives less energy when it is cloudy.
3. Low power output.
4. Requires large areas.
5. The solar cells are expensive.
Look at the data booklet: 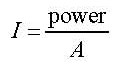 where
where
I = intensity
A = area
so the intensity is defined as the power of the radiation divided with the area that it hits. It varies with the latitude and the time of day and the time of year.
Look at the data booklet: 
so the albedo is the fraction of the incoming power that is reflected away.
8.6 - Hydroelectric power
Hydroelectric power plant
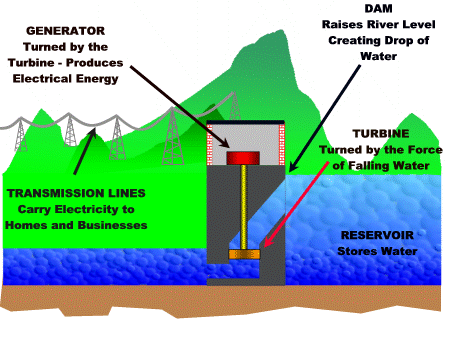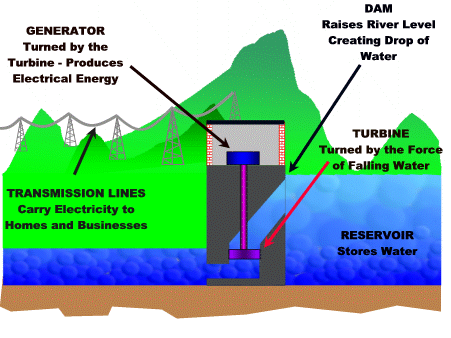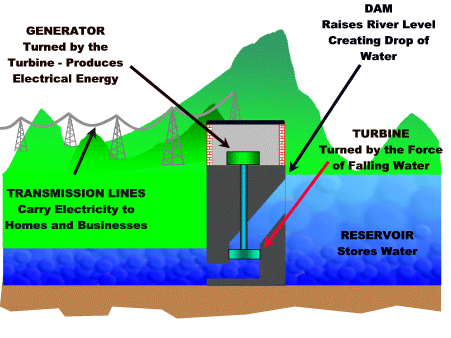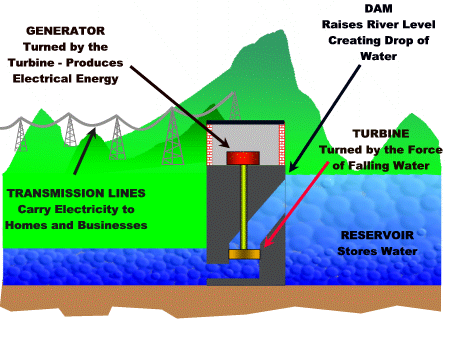
The falling water from a dam is used to turn a turbine that is connected to a generator.
For more detail click on this link.
Advantages of hydroelectric power
1. No cost of fuel.
2. Will never be exhausted.
3. Clean.
Disadvantages of hydroelectric power
1. Needs a river.
2. Destroys the environment when a dam is built.
3. The initial cost of building a dam is high.
Calculating the power of a hydroelectric station
One gets the power from the change of the potential energy of the water per unit of time
as it falls down and hits the turbine:
P = mgh / t where
P = power
m = mass of the water
g = gravitational constant
h = the height that the water falls
t = time
we can modify this formula by using the formula for density (ρ = m/V = 1000 kg/m3):
P = mgh / t = ρVgh / t
the quantity V/t is called the volume flow rate.
8.7 - Wind power
A wind power plant is simply a propeller that is turned by the wind
and the propeller axis is then turning an electric generator.
Advantages of wind power
1. No cost of fuel.
2. Will never be exhausted.
3. Clean.
Disadvantages of wind power
1. Works only when there is wind.
2. Low power output.
3. Large maintenance costs.
4. Ruins the view of the landscape.
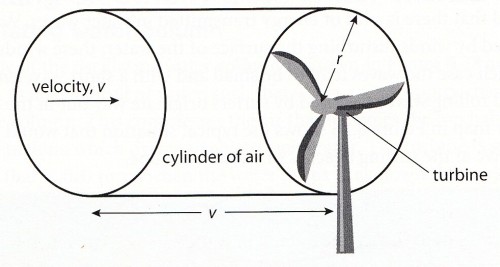
Calculating the power of a wind power station
The power can be calculated from the kinetic energy of the air moving through the propeller:
Look at the data booklet: 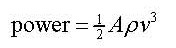 where
where
ρ = density of air (1.2 kg/m3)
A = area of the propeller
v = velocity of the wind
8.8 - Wave power
Wave power plant
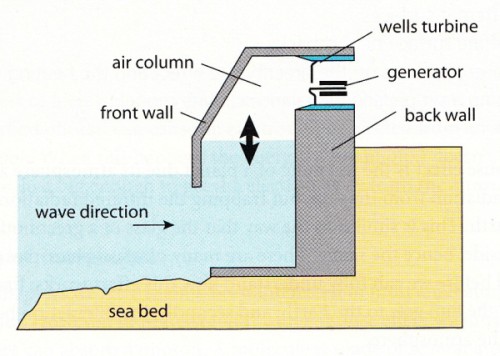
The incoming waves push the air up past a turbine (propeller) which turns a generator.
When the wave goes back out again the air is sucked down and that makes the turbine move in
the other direction.
Advantages of wave power
1. No cost of fuel.
2. Will never be exhausted.
3. Clean.
4. Reasonable energy density.
Disadvantages of wave power
1. Works only in areas with large waves.
2. Waves changes which makes it difficult to use them.
3. Large maintenance costs.
4. Large installation costs.
5. Difficult to transport the power to the consumer.
6. Difficult to use low frequency waves with high frequency turbines.
Calculating the power of a waves
The power per unit length of a wave is:
Look at the data booklet: 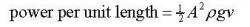 where
where
ρ = density of water (1000 kg/m3)
g = gravitational constant
A = amplitude (height) of the waves.
v = velocity of the waves.
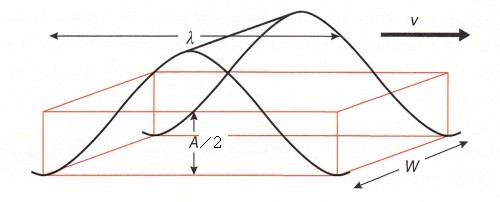
The speed (v) of a wave is given by:
Look at the data booklet: 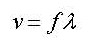 where
where
f = is the frequency of the waves.
λ = the wavelength.
Remember that you can calculate the frequency from the time period (T):
f = 1/T
8.9 - Greenhouse effect
If the sun radiates Isun=3.9x1026 W then the intensity of
the light hitting a planet on a distance d is
I = Isun / 4πd2
GASES
Excitation of atoms:
When a photon interacts with single atoms it can push the electrons up to higher
energy levels. This can only happen if the photon has exactly the energy that
corresponds to the difference between energy levels in the atoms.
Ionization of atoms:
If the photon energy is high enough it can completely kick out the electrons from
the atoms (no special energy level is needed just an energy high enough).
Ionization of molecules:
Photon with the right energy can be absorbed by gas molecules and make the
molecules vibrate.
SOLIDS
Solids consists of many atoms that interacts with each other. The electrons can
now have many different energies and this means that solids can absorb photons
with many different energies.
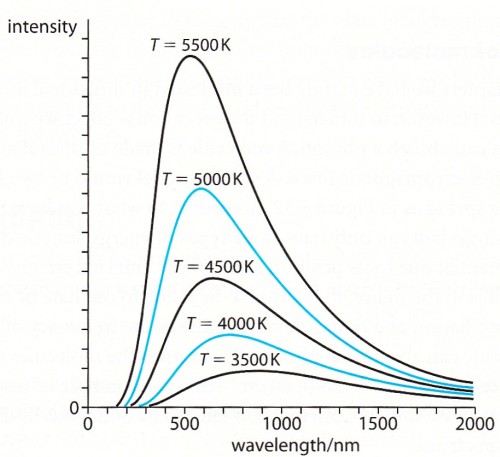
Black body radiation
While a gas will only absorb light with several wavelengths, a black solid object can absorb all
wavelengths.
It is the same thing when gases and objects emits (send out) radiation. Gases send out light at
only certain wavelengths but black solid objects can emit radiation at all wavelengths. When a
black solid object is heated to a temperature T it will send out radiation with a continuous spectrum
that depends on T. This spectrum of radiation is called back body radiation.
Black body radiation spectrum
Stefan-Boltzmanns law
Look at the data booklet: 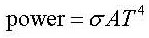
Where the constant σ can also be found in
the data booklet:
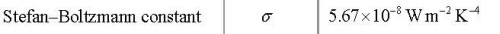
The Stefan-Boltzmann law is obtained by integrating the curves in the diagram above.
It gives the total power emitted by a black body with surface area A if it has the temperature T.
Stefan-Boltzmanns law
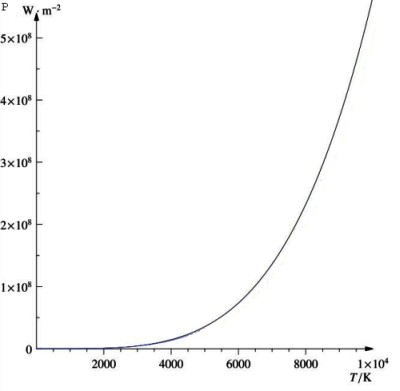
Emmisivity
Emmisivity (e) is a parameter that tells us how close a solid is to a perfectly black object.
It can have values between 0 and 1 where e=0 is not a black object at all and e=1 is a perfectly
black object. If an object is not perfectly black then one has to modify the Stefan-Boltzmann law.
Look at the data booklet: 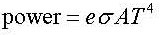
The greenhouse effect
When the solar radiation hits the earth's atmosphere some of the radiation will be absorbed
by Ozon and some of the infrared radiation will be absorbed by greenhouse gases such as
CO2 and H2O. This will heat the atmosphere up.
The rest of the radiation will hit the ground. Some of this radiation will be
reflected out to space but the rest of the radiation will be used to heat the ground.
The heated ground will now send out new infrared radiation like a black body.
This infrared radiation from the ground can now be absorbed by the greenhouse gases. Without
these the radiation from the ground would go back out to space.
The surface heat capacity
The surface heat capacity Cs is the amount of heat (Q)
in an area of 1 m2 that is needed to
raise the temperature of the ground with 1 Kelvin.
Look at the data booklet: 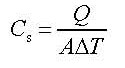 where
where
A = Area of the ground.
Q = Heat energy.
ΔT = Change in temperature.
Look at the data booklet: 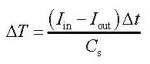 where
where
ΔT = Change in temperature.
Cs = the surface heat capacity.
Iin = the incoming intensity of the solar radiation.
Iout = the outgoing intensity of the solar radiation.
t = time
8.10 - Global warming
Solar flares and sunspots
The sun will radiate more when there are lots of sunspots.
Earth's orbit
The distance between the sun and the earth is not constant and this will change the amount of radiation hitting the earth.
Earth's axis
There are changes of the axis in relation to the sun and this change the amount of radiation.
The enhanced greenhouse effect is when the amount of greenhouse gases in the atmosphere increases.
The amount of radiation absorbed by the atmosphere is then also increased. The amount of energy leaving the earth
is reduced. And the temperature of the Earth is increased.
Greenhouse gases
The most important greenhouse gases are water (H2O), carbon dioxide (CO2),
methane (CH4) and nitrous oxide (N2O).
Measurements over thousands of years
Every year a new layer of ice is deposited in Antarctica. From the different isotopes of hydrogen in
the ice one can determine the temperature when the layer was made. From bubbles of air in the ice one
can determine the CO2 levels. There is a clear correlation between CO2 levels
in the ice-layers and the temperature.
Measurements over the last 50 years
The last 50 years one has been able to measure the average temperature and the average CO2 levels
in the atmosphere directly. They have both increased.
The burning of fossil fuels have increased a lot the last 50 years and this produces CO2 while
forests that absorb CO2 have been cut down.
8.11 - What might happen
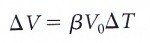 where
where
β = the coefficient of volume expansion.
ΔT = Change in temperature of a liquid.
V0 = the original volume of the liquid before the temperature changed.
ΔV = Change of volume of a liquid.
So β is a constant that tells how much a liquid will expand when the temperature change
with ΔT.
Rise in sea levels
This is caused by an expansion of the water in the oceans as it gets warmer and the melting
of the ice caps.And the melting of the ice on Antarctica and Greenland (but not on the northpole since that ice
is floating on an ocean). If this leads to changes in ocean currents this could change weather patterns a lot.
Change in weather
It is expected that countries near the equator will get hotter and countries in the northern hemisphere will
get wetter.
Solutions
1. Power plants with higher efficiency.
2. Replacing coal and oil with natural gas.
3. Use power stations to both produce electricity and heat homes.
4. Use more renewable energy and nuclear power.
5. use vehicles that runs on both petrol and electricity.
6. capture carbon dioxide from power plants and store it undergrounds.



